Abstract
- Pancreatic Ductal Adenocarcinoma (PDAC) remains one of the deadliest cancers worldwide, largely due to its silent progression and late diagnosis. Most patients are identified at an advanced stage, when therapeutic options are limited and survival rates are extremely low. Detecting precursor lesions, such as Pancreatic Intraepithelial Neoplasia (PanIN), represents a crucial opportunity to intervene earlier and improve patient outcomes.
- Our project, ExoSPY, introduces an innovative theranostic strategy that leverages engineered extracellular vesicles (EVs) to both detect and treat precancerous pancreatic lesions. These EVs are designed to target Claudin-4 (CLDN4), a tight junction protein overexpressed and abnormally accessible in PanIN and PDAC tissues.
- By integrating a cCPE-Lamp2b fusion protein onto the EV surface, we enable specific targeting of precancerous cells. These vesicles also carry components allowing MRI-based visualization and X-ray–activated therapeutic response, offering a dual-function platform for early detection and localized treatment.
- Through ExoSPY, we aim to provide a non-invasive, precise, and scalable synthetic biology approach to detect pancreatic cancer earlier before it becomes invasive and open new perspectives in cancer theranostics.
Oncology
- Among the diverse scientific fields represented in iGEM, our team was naturally drawn to oncology, guided by both our academic backgrounds and personal experiences. Cancer remains one of the leading global health challenges, affecting millions of lives each year. According to the World Health Organization and the International Agency for Research on Cancer (IARC), over 20 million new cancer cases and approximately 10 million cancer-related deaths were recorded worldwide in 2022, a number projected to rise to more than 30 million new cases annually by 2040.
- These statistics not only highlight the immense burden of cancer on public health systems but also emphasize the urgent need for innovative diagnostic tools and therapeutic approaches.
- Despite major advances in precision medicine and immunotherapy, many cancers are still detected at advanced stages, where treatment options are limited and survival rates are drastically reduced. Our project seeks to contribute to this ongoing global effort by exploring new synthetic biology strategies to improve early detection and intervention in oncology.
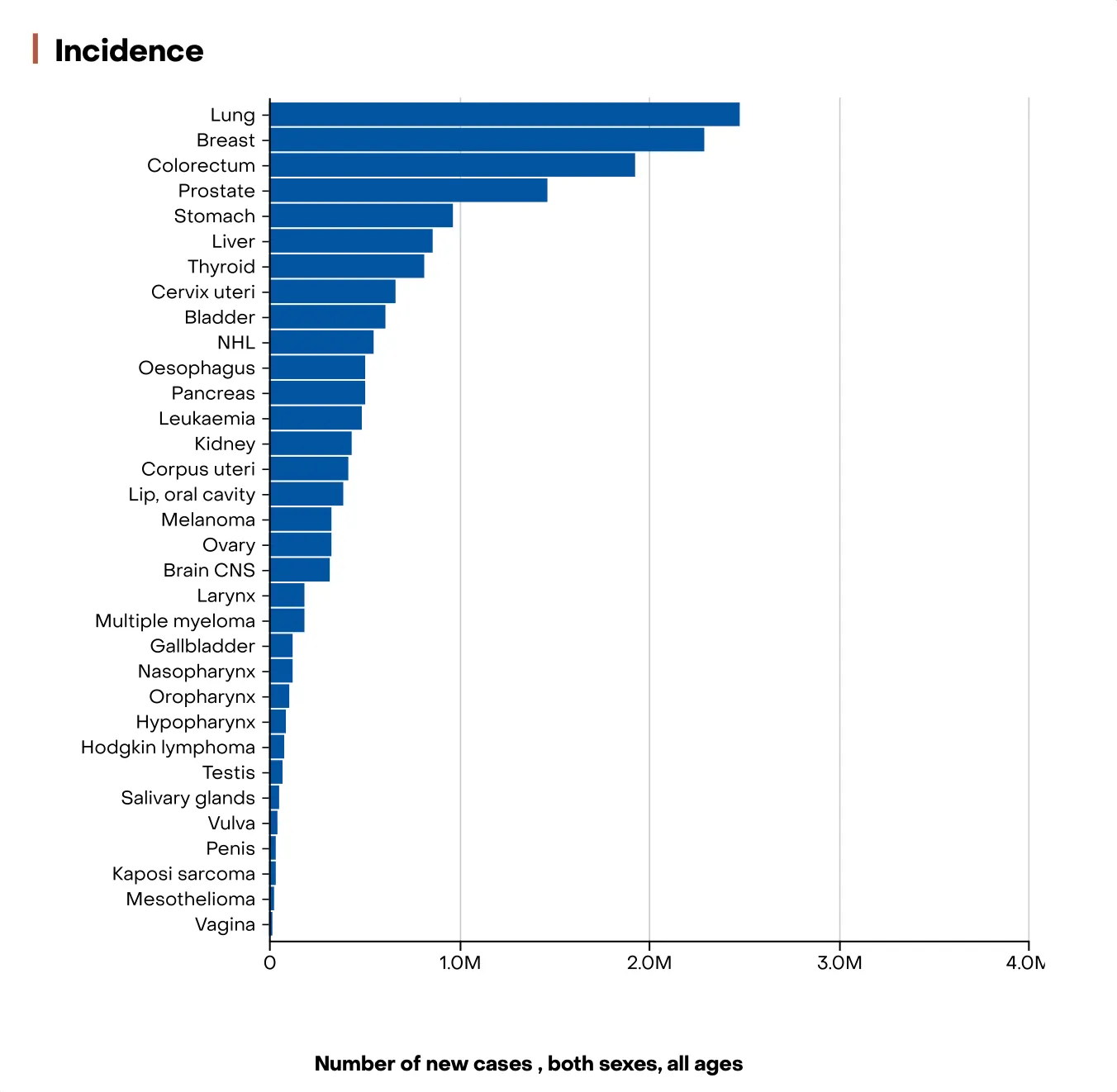
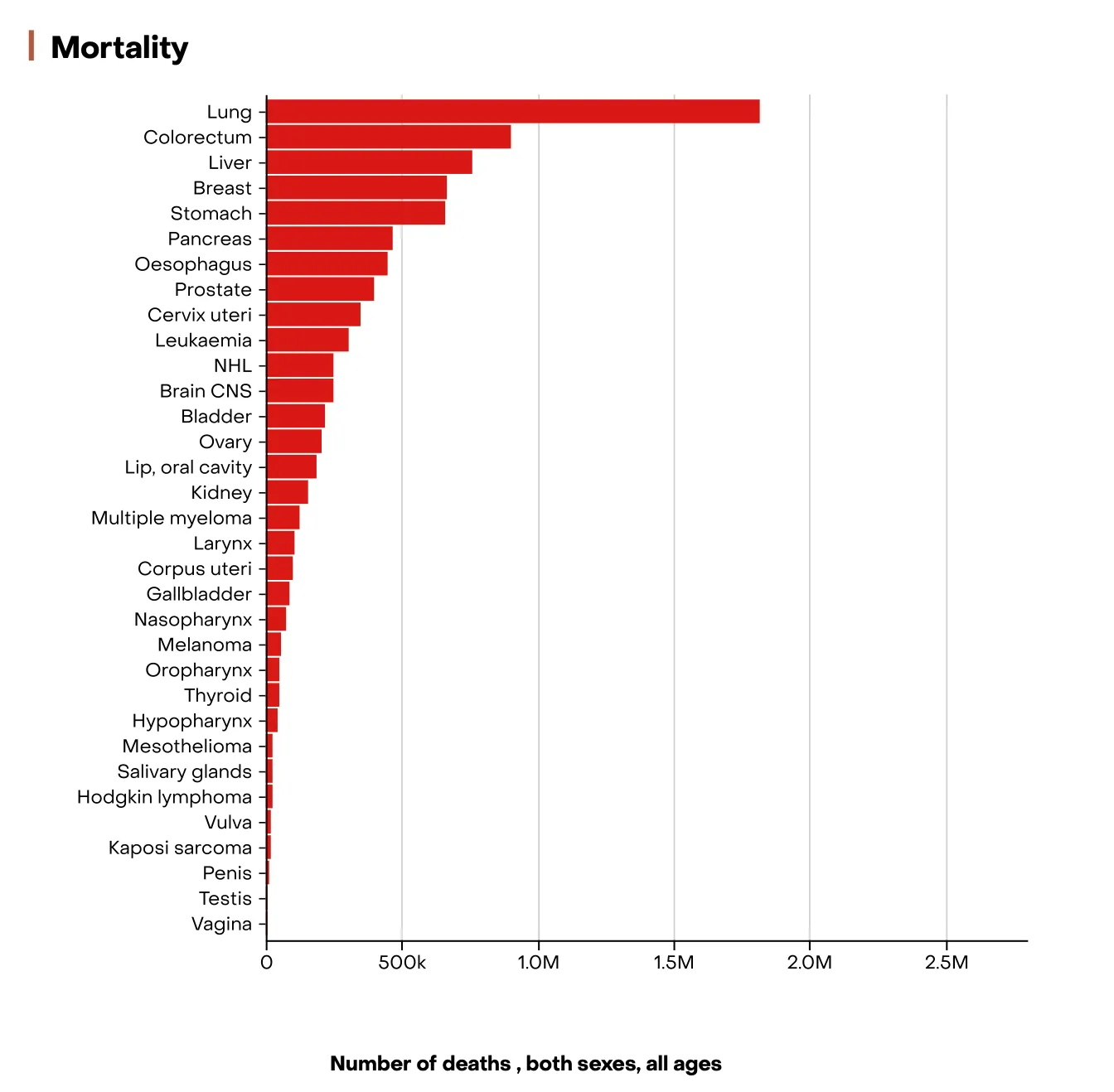
Figure 1: Graph showing cancer incidence and mortality worldwide in 2022
PDAC the Problem and Its Impact
- Pancreatic cancer or Pancreatic Ductal Adenocarcinoma is one of the deadliest malignancies worldwide, largely due to its asymptomatic progression and late-stage diagnosis. In 2022, an estimated 510,992 new cases and 467,409 deaths were reported globally, making it the seventh leading cause of cancer-related deaths across both sexes.
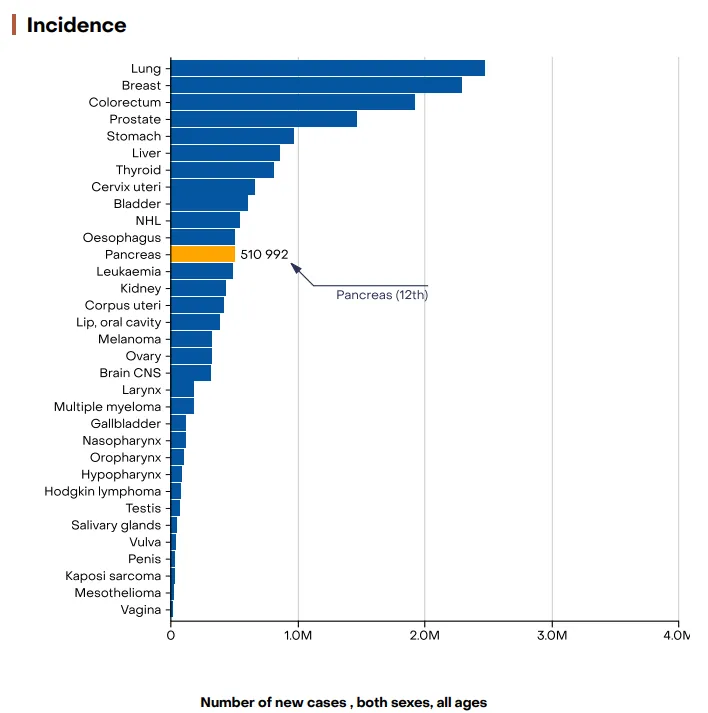
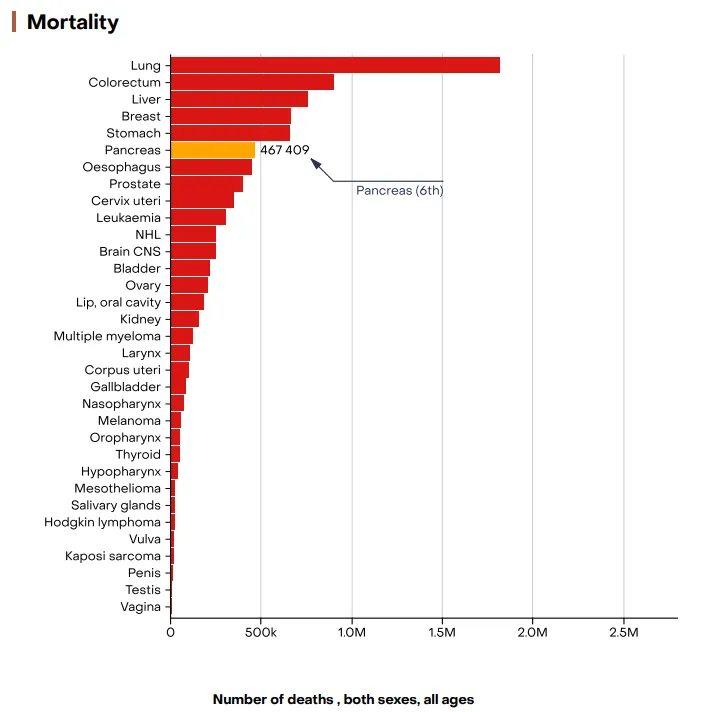
Figure 2: Graph showing the incidence and mortality of pancreatic cancer worldwide in 2022
- Despite advances in oncology, pancreatic cancer remains associated with extremely poor outcomes. The 5-year overall survival rate is approximately 10%, dropping below 5% for patients diagnosed with metastatic disease (Acobiom, 2023). The median survival for advanced cases is often less than one year, reflecting the urgent need for earlier and more accurate diagnostic methods.
- Over the past decades, the incidence of pancreatic cancer continues to rise. Pancreatic Ductal Adenocarcinoma (PDAC) could become the second leading cause of cancer mortality in the years 2030-2040. This upward trend is particularly pronounced in high-income countries, where aging populations and lifestyle factors contribute to the increased burden.
Focus on PanIN
- Histologically, PDAC often evolves from Pancreatic Intraepithelial Neoplasia (PanIN) microscopic precancerous lesions that accumulate stepwise genetic alterations before becoming invasive.
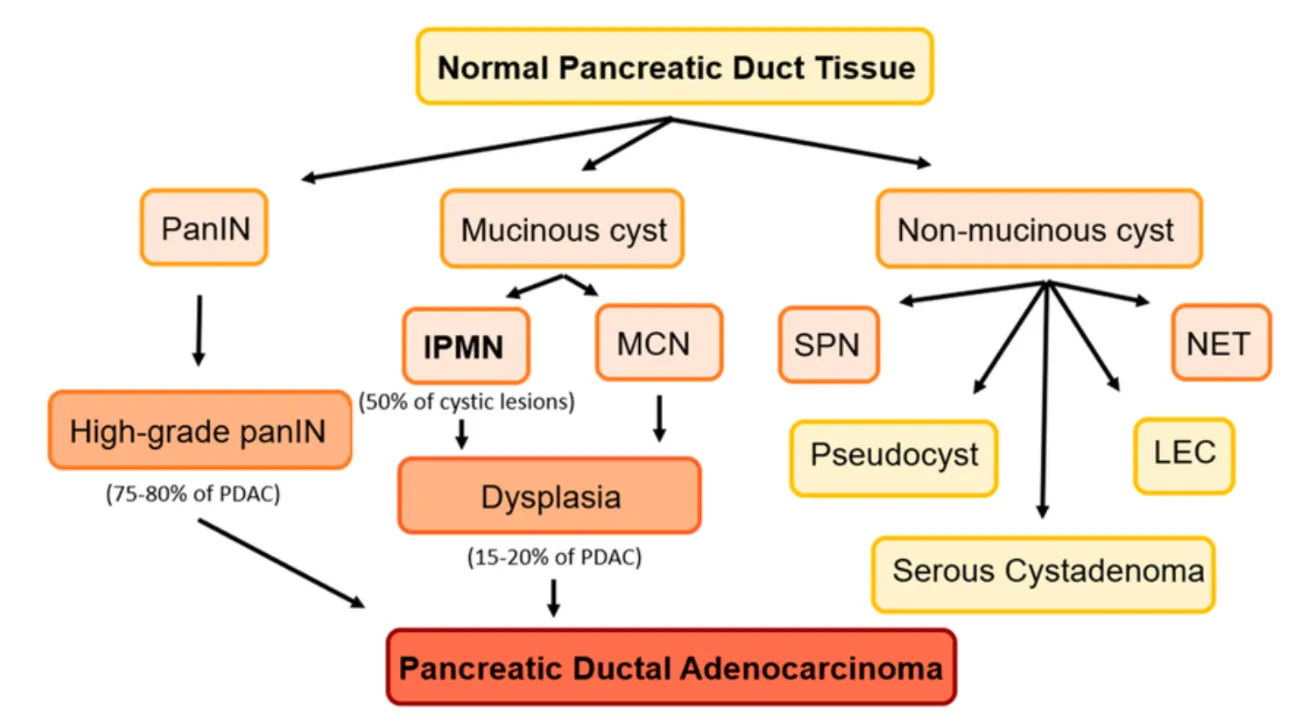
Figure 3: Precancerous lesions of the pancreas. Mucinous cysts: (IPMNs) Intraductal papillary mucinous neoplasms and mucinous neoplasms (MCN). Non-mucinous cysts: serous pseudopapillary neoplasms (SPN), lymphoepithelial cysts (LEC), and cystic neuroendocrine tumors (NET).
PanINs are microscopic lesions (not visible by standard imaging) and are classified by increasing histological dysplasia. The major grades are:
- PanIN-1A / 1B (low-grade): minimal atypia, mucinous epithelial cells, relatively benign architecture
- PanIN-2 (intermediate grade): increased nuclear abnormalities, some crowding, loss of polarity
- PanIN-3 (high-grade): marked cytologic atypia, architectural distortion, budding, possible luminal necrosis
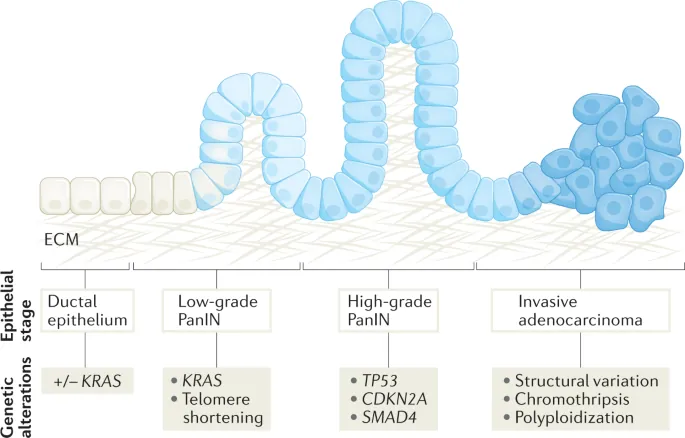
Figure 4: Stepwise Genetic and Morphological Progression from Normal Ductal Epithelium to Invasive Pancreatic Adenocarcinoma
-
We chose to focus on PanIN lesions because they are the most frequent and earliest form of
pancreatic precancerous changes. As the primary pathway leading to PDAC, they represent the
key target for early detection and intervention strategies. Detecting PanINs could allow
clinicians to diagnose pancreatic cancer before it becomes invasive, drastically improving
survival rates.
[1]
Reference 1: Early neoplastic lesions of the pancreas: initiation, progression, and opportunities for precancer interception Brian A. Pedro1 and Laura D. Wood1.
[2] Reference 2: Pancreatic Ductal Adenocarcinoma and Its Precursor Lesions Histopathology, Cytopathology, and Molecular Pathology Bing Ren ∙ Xiaoying Liu ∙ Arief A. Suriawinata.
Our Solution : ExoSPY
- Our project proposes an innovative theranostic strategy that combines magnetic resonance imaging (MRI)–based detection with X-ray–triggered therapy. To tackle the growing global burden of pancreatic cancer, we have engineered genetically modified extracellular vesicles (EVs) capable of specifically targeting precancerous pancreatic cells.
-
EVs are small extracellular vesicles, with a diameter between 30 and 150 nm, produced by
most eukaryotic cells. They are generated from late endosomes through the formation of
multivesicular bodies (MVBs), which then fuse with the plasma membrane to release exosomes
into the extracellular environment. These vesicles reflect the physiological or pathological
state of the cell of origin. They transport proteins, lipids, DNA, messenger RNA and
microRNAs. Circulating exosomes are present in many biological fluids, such as blood, urine,
saliva and cerebrospinal fluid.
[8]
Reference 8:
« Welsh, Joshua A., Deborah C. I. Goberdhan, Lorraine O’Driscoll, Edit I. Buzas, Cherie Blenkiron,
Benedetta Bussolati, Houjian Cai, et al. 2024. “Minimal Information for Studies of Extracellular
Vesicles (MISEV2023): From Basic to Advanced Approaches.” Journal of Extracellular Vesicles
13 (2): e12404. »
They are able to cross biological barriers such as the blood-brain barrier, thanks to their nanometric size and mechanisms of endocytosis or membrane fusion. [9] Reference 9: « Abdelsalam, Manal, Munazza Ahmed, Zaynab Osaid, Rifat Hamoudi, et Rania Harati. 2023. “Insights into Exosome Transport through the Blood–Brain Barrier and the Potential Therapeutical Applications in Brain Diseases.” Pharmaceuticals 16 (4): 571. » Extracellular vesicles are the subject of numerous studies, particularly in clinical research, mainly as therapeutic vectors or immunomodulatory agents. Although no exosome-based treatments have yet been approved, preliminary results are promising, particularly in oncology, neurology and immunotherapy. - In our project, these EVs are designed to act as dual-function nanocarriers: they enable non-invasive imaging through MRI contrast enhancement, while simultaneously serving as vectors for localized therapeutic action upon X-ray activation. This dual approach offers a promising pathway to detect pancreatic cancer earlier and treat it more precisely, potentially improving patient prognosis and minimizing damage to surrounding healthy tissues.
- Our engineered extracellular vesicles are produced from HEK293 cells, a widely used and well-characterized mammalian cell line known for its efficiency in exosome production and recombinant protein expression. Using this system ensures the generation of biocompatible, scalable, and safe vesicles, making it an ideal platform for developing innovative theranostic tools.
- To achieve this precise targeting and imaging capability, we designed a custom fusion protein that decorates the surface of these vesicles.
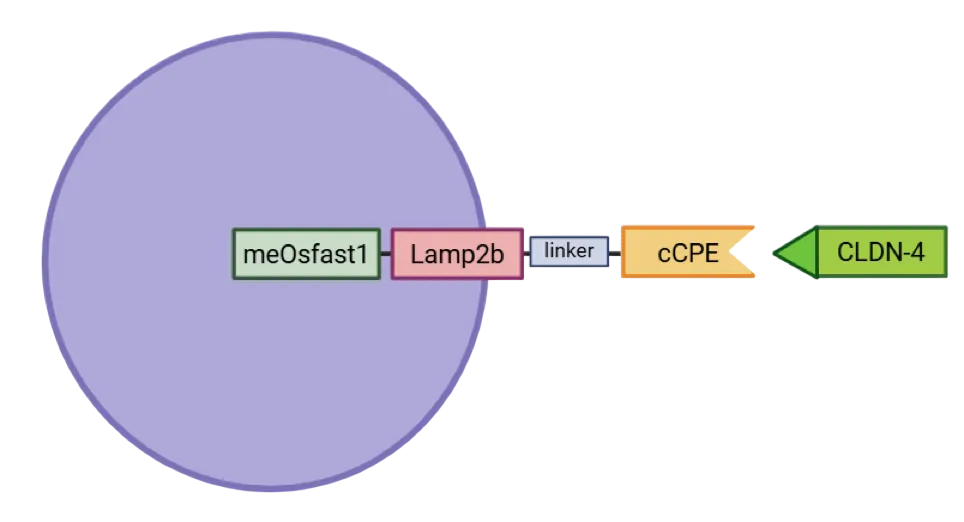
Figure 5: The cCPE-Lamp2b-linker-meOsfast1 fusion protein. This protein is produced by synthetic biology. On the extravascular side, c-CPE194-317 is spaced by a Lamp2b linker that is transmembrane, allowing anchoring in the membrane. On the intravascular side, the fluorescent protein mEosfast1. This hybrid protein is expressed in an exosome, shown schematically here.
The construct integrates several functional domains:
- cCPE: a enterotoxin having an high affinity to Claudin-4 (CLDN4), a tight junction protein frequently overexpressed in pancreatic ductal adenocarcinoma (PDAC).
- Linker to preserve their individual structure and function.
- Lamp2b: an exosomal membrane protein ensuring vesicle anchoring
- meOsfast1: a fluorescent protein enabling real-time imaging and vesicle tracking.
Together, they provide our vesicles with both specificity toward cancer cells and diagnostic visibility, creating an integrated theranostic platform.
Porosity of Healthy vs. Cancerous Tissues
-
The accessibility of epithelial tissues differs between healthy and cancerous states [6]
Reference 6:
Claudin-4: A New Molecular Target for Epithelial Cancer Therapy ». s. d. Consulté le 9 juin 2025.
. In healthy tissue, epithelial cells form a tight barrier through tight junction proteins, including CLDN4 (Figure 2A). These junctions maintain cell adhesion and polarity, selectively regulate the passage of ions and growth factors, and preserve a homeostatic cellular environment. They also play a key role in activating intracellular signaling pathways that contribute to tumor progression and metastasis. - When a cell becomes cancerous, it loses its polarity and physiological architecture. To compensate for this loss of cohesion, the cell overexpresses tight junction proteins such as CLDN4, which helps maintain a certain level of organization, albeit in a disordered manner. Consequently, in cancerous tissue, cells are less tightly connected, and tight junction proteins become more accessible due to this cellular disorganization (Figure 2B). Therefore, CLDN4 overexpression may represent an early event in pancreatic ductal adenocarcinoma (PDAC) tumorigenesis.
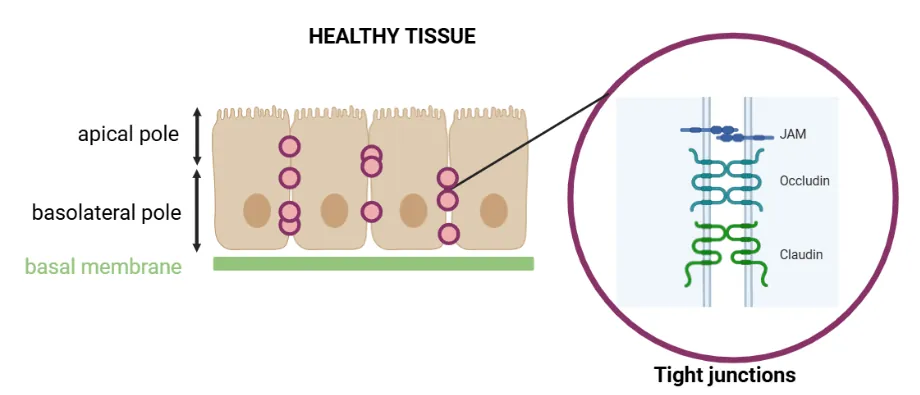
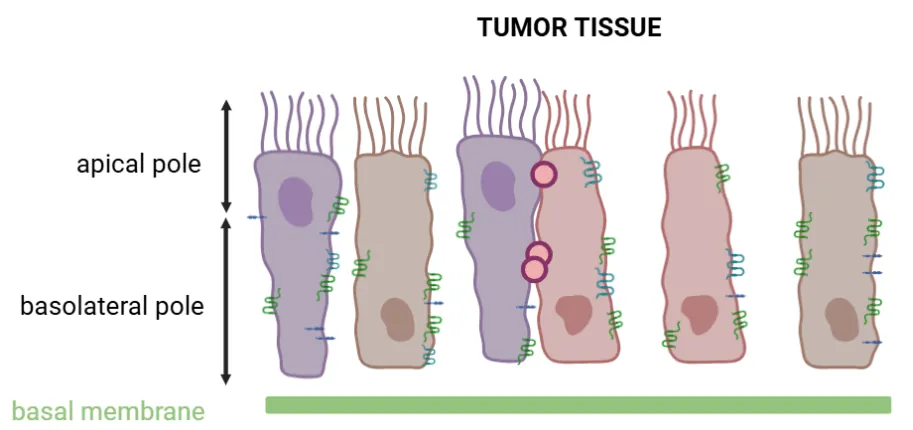
Figure 6: Schematic representation of healthy (A) and tumor (B) epithelial tissues showing tight junction protein accessibility
The Claudin Family: Focus on Claudin-4
- Claudins are a family of integral membrane proteins that constitute the tight junctions, the major intercellular junctions responsible for regulating paracellular permeability and maintaining epithelial cell polarity by separating the apical and basolateral membrane domains. In mammals, the claudin family currently includes 27 members, several of which are expressed in various tissues.
- Claudins are tetraspan transmembrane proteins of approximately 25 kDa, comprising four transmembrane domains (TM1–4), cytoplasmic N- and C-terminal ends, and two extracellular loops (ECL1 and ECL2). ECL1 contains four β-strands and one extracellular helix (ECH), as well as a β-strand from the TM3 domain exposed at the cell surface. Each ECL also includes a variable region (V1 and V2) (Figure 1).
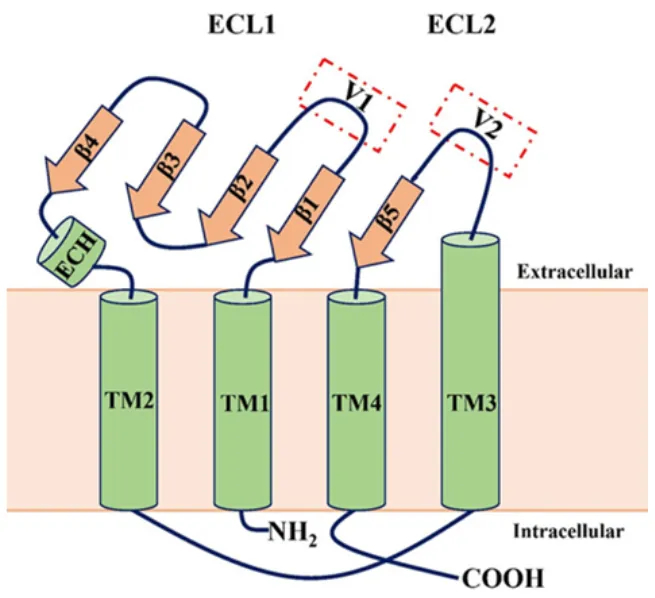
Figure 7: General schematic of claudin structure. Claudins are tetraspan transmembrane proteins with four transmembrane domains (TM1–4) and two extracellular loops (ECL1 and ECL2).
- Some claudins have been explored as therapeutic targets in oncology. For instance, zolbetuximab, a monoclonal antibody targeting claudin-18.2, induces immune cell-dependent cytotoxicity against cancer cells expressing this protein—an innovative therapeutic approach given the overexpression of claudin-18.2 in digestive cancers [4] Reference 4: « Tight Junction Proteins Claudin-3 and Claudin-4 Are Frequently Overexpressed in Ovarian Cancer but Not in Ovarian Cystadenomas | Clinical Cancer Research | American Association for Cancer Research ». s. d. Consulté le 9 juin 2025.
- Other studies have shown that claudin-4 (CLDN4) is a promising candidate biomarker for the detection of pancreatic, ovarian, prostate, gastric, and bladder cancers [2,3] Reference 2: « Pancreatic Ductal Adenocarcinoma and Its Precursor Lesions Histopathology, Cytopathology, and Molecular Pathology Bing Ren ∙ Xiaoying Liu ∙ Arief A. Suriawinata. ». Reference 3: « Claudine 18.2 : Nouvelle Cible Thérapeutique Dans Les Cancers Digestifs ». 2024. Bulletin Du Cancer 111 (12): 1133‑41 ». . This detection relies on the overexpression of CLDN4 in tumor tissues, particularly in PDAC, and is also observed in precancerous lesions such as PanINs.
Why Claudin-4?
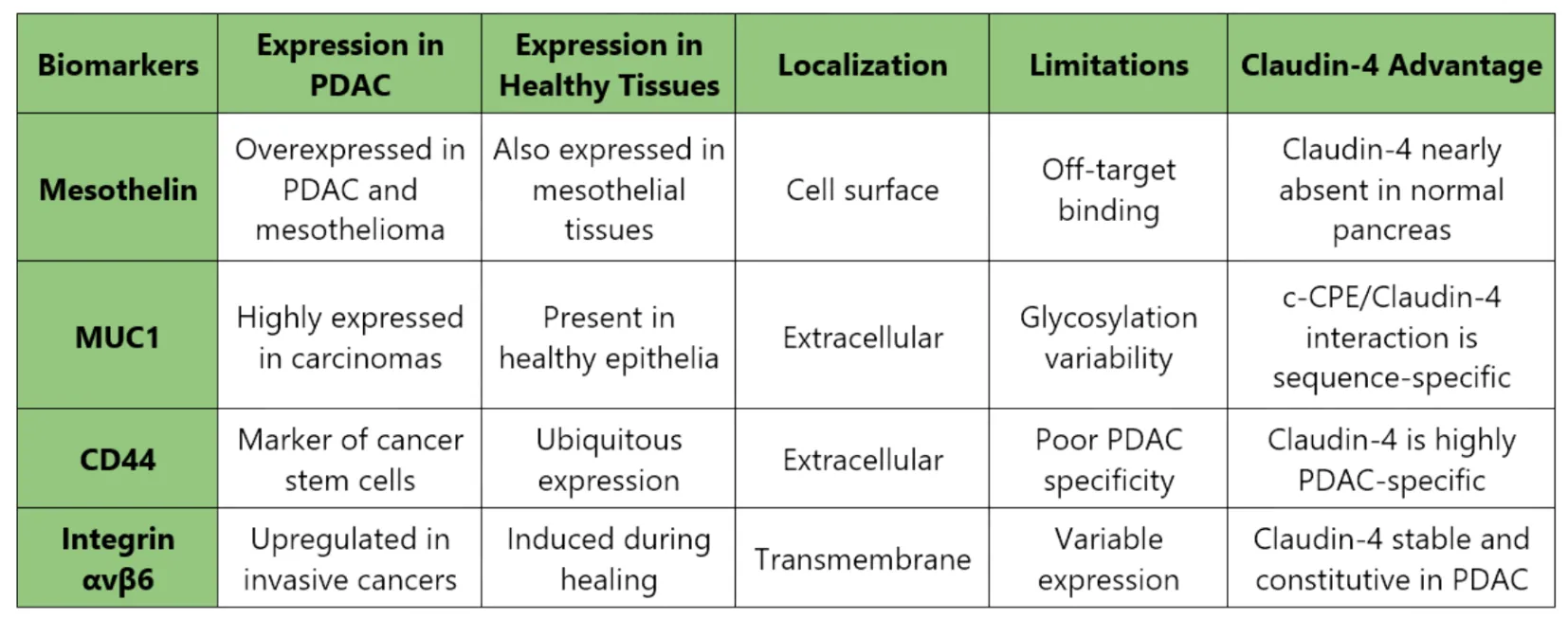
Figure 8: Comparative Analysis of Pancreatic Ductal Adenocarcinoma (PDAC) Biomarkers and Advantages of Claudin-4 Targeting
Key reasons for choosing Claudin-4:
- Highly specific to PDAC (>90% of tumors)
- Accessible due to junction disorganization in tumor cells
- Has a natural ligand, c-CPE, that binds with nanomolar affinity
- Low off-target risk in normal tissues
- Crystal structure available enabling rational design
Interaction Between CLDN4 and cCPE
- Recent studies have revealed an interaction between CLDN4 and the Clostridium perfringens enterotoxin (CPE). CPE is a polypeptide toxin—a linear chain of amino acids linked by peptide bonds, of approximately 35 kDa and 319 amino acids. CPE consists of a C-terminal domain responsible for binding to the CLDN4 receptor, and an N-terminal domain involved in oligomerization, membrane insertion, and pore formation.
- CLDN4 is described as a high-affinity receptor for CPE, enabling an irreversible interaction between the C-terminal domain of CPE and the extracellular loops ECL1 and ECL2 of CLDN4 [7] Reference 7: Tight Junction Proteins Claudin-3 and Claudin-4 Are Frequently Overexpressed in Ovarian Cancer but Not in Ovarian Cystadenomas | Clinical Cancer Research | American Association for Cancer Research. . The region of CPE recognizing the ECL2 domain of CLDN4 spans amino acids 194–317.
- In our project, we aim to exploit this binding property of CPE to target accessible CLDN4 in early-stage cancerous tissues. To deliver CPE to these tissues, we will use exosomes as a transport system.
Bonus: The Logo - Why and How?
- For us, it was essential that our logo could speak to anyone! That’s why its design took us many days, weeks, even months, to complete.
- ExoSPY: a name that is both striking and meaningful. It evokes exosomes acting as spies, within the context of pancreatic cancer.
- We wanted a logo that truly represented who we are, something that reflects each member of our team. This idea guided us throughout the entire creative process. The arrival of Yussera in mid-May 2025 was a tremendous help in shaping all the concepts that were flowing from our minds.
- What an amazing step in our journey! As scientists, we wanted to create something impactful, clear, and even a little fun.
✨ ExoSPY: Exosomes Solution for Pancreatic, You Need! ✨
