Cycle 1: Establishing a Robust HEK293 Cell Platform
A) Design
- We planned to use HEK293 cells as our EV-producing system. Both adherent (HEK293T) and suspension (HEK293F) cultures were tested to identify the most suitable format for large-scale EV production.
B) Build
Two culture systems were set up:
- HEK293T (adherent, grown in DMEM)
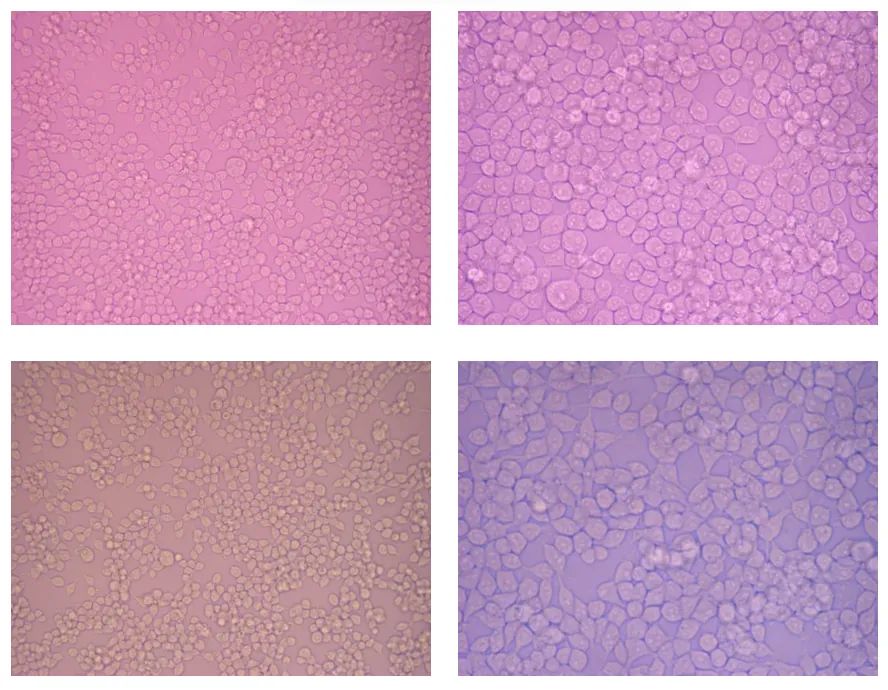
Figure 1: P HEK293T under an inverted microscope with X20 magnification at the left and X40 magnification at the right. Pictures from the top half are from 24wells culture plate, and the bottom is from T150 Flask
- HEK293F (suspension, grown in serum-free medium)
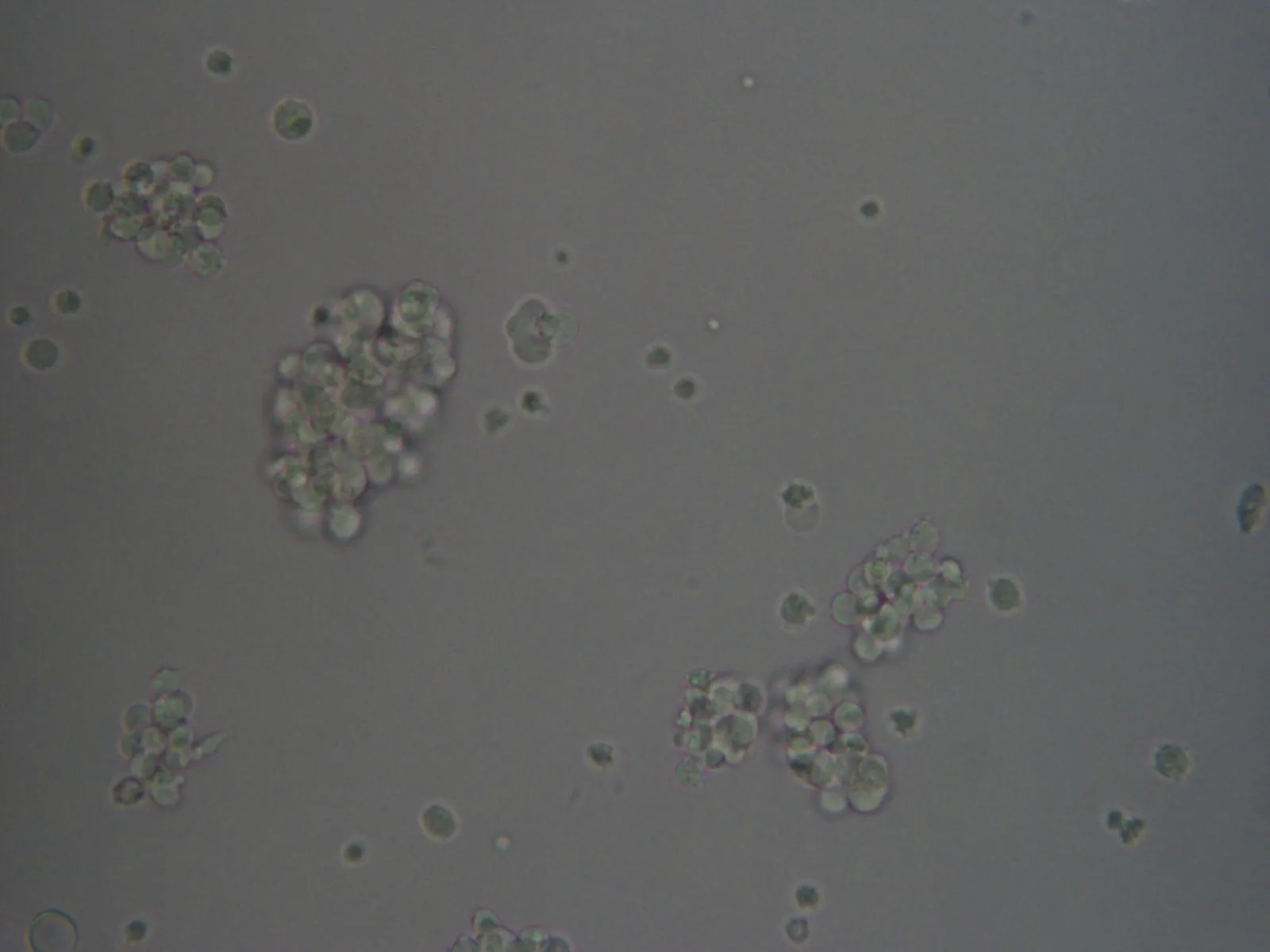
Figure 2: HEK293F culture under an inverted microscope with X20 magnification
C) Test
- HEK293F cultures rapidly lost viability due to insufficient agitation, leading to cell sedimentation and oxygen limitation. In contrast, HEK293T cells remained healthy and adherent with high viability.
D) Learn
- We concluded that adherent HEK293T cells were better suited for consistent transfection and EV production. Suspension systems were discontinued, and subsequent experiments focused on HEK293T.
Cycle 2: Optimizing Transfection Efficiency
A) Design
- To maximize EV production and labeling, we needed to optimize HEK293T transfection efficiency using different reagents and DNA:reagent ratios.
B) Build
- We tested multiple transfection reagents (Dreamfect®, ICAFectin®, PEI®, jetPEI®) using a GFP plasmid (pcDNA3.1-GFP) as a reporter.
C) Test
- Dreamfect® achieved the highest transfection rate (~27–30%), while other reagents gave poor results. We tested these conditions twice, several weeks apart, with the pcDNA3.1 plasmid and with the plasmid encoding GoldenEye.
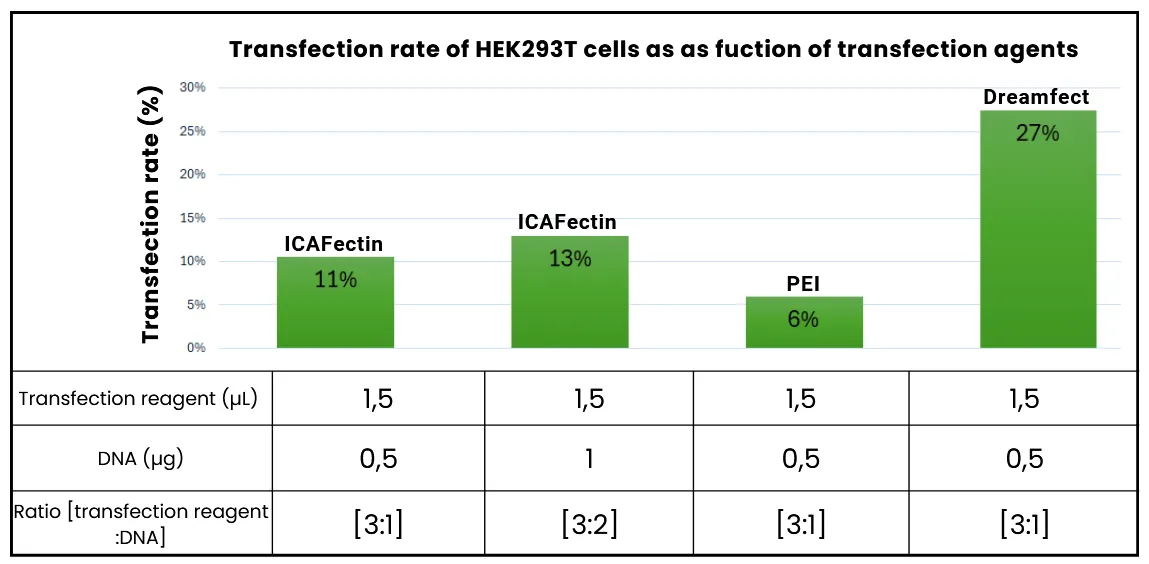
Figure 3: Histogram of HEK293 cell transfection rates depending on transfection agents, under the best conditions observed for each agent. JetPEI® is not included here because the transfection rates obtained with this agent are zero
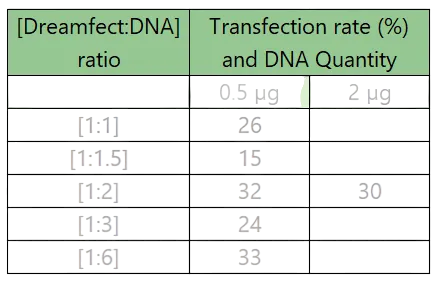
Table 1: Effect of Dreamfect:DNA ratio on transfection rate at different DNA quantities
D) Learn
- Dreamfect® was selected for further experiments using the pexpIDT plasmid ("GFP-Lamp2b.trunc-Linker4-cCPE"). The optimal condition was a 1:2 Dreamfect:DNA ratio with 2 µg DNA per well.
Cycle 3: Testing Engineered EV-Cell Interactions
A) Design
- Evaluate whether the engineered EVs could specifically bind to or be internalized by pancreatic cells (BxPC3, MIA PaCa-2, and PANC-1) via the cCPE–Claudin-4 interaction.
B) Build
- Cells were incubated with modified EVs at multiple time points (30 min to 6 h) under continuous agitation to promote contact.
C) Test
- A slight increase in FITC fluorescence was observed at early time points, suggesting initial EV-cell interactions. However, prolonged agitation caused massive cell death, making results non-interpretable.
D) Learn
- Agitation induced mechanical stress, destroying cells and masking real interactions. Future iterations will use gentle EDTA detachment and static incubation to preserve viability and assess specific EV uptake accurately.
Cycle 4: Target Validation and Characterization
A) Design
- Confirm that the pancreatic cell lines express the target receptor, Claudin-4 (CLDN4).
B) Build
- Western blotting was performed on MIA PaCa-2, BxPC3, and PANC-1 lysates.
C) Test
- All three cell lines showed CLDN4 bands between 25–37 kDa, confirming expression and validating them as suitable target models.
D) Learn
- This confirmed that the observed EV targeting (early fluorescence shift) was biologically plausible. Future work will focus on optimizing incubation and detection to confirm specific binding.