Project Description
Background
Cancer is the second leading cause of death globally. According to statistics from the World Health Organization (WHO), there were over 20 million new cancer cases and approximately 10 million cancer-related deaths worldwide in 2022. Among these, breast cancer has become the most commonly diagnosed cancer, accounting for 11.7% of all new cases. As the Special Administrative Region of China with the highest population density, Macao exhibits distinct regional characteristics in its cancer incidence patterns. Based on the latest cancer registry report released by the Macao Health Bureau, malignant tumors have become the leading cause of death among local residents, representing 35.7% of all deaths. The disease burden of breast cancer is particularly prominent. In terms of epidemiological characteristics, the age-standardized incidence rate of female breast cancer in Macao reaches 68.5 per 100,000, significantly higher than the global average (46.3 per 100,000), ranking first among all female malignancies. Breast cancer accounts for 28.3% of all new cancer cases in women, meaning one in every four female cancer patients is diagnosed with breast cancer. It is noteworthy that breast cancer incidence in Macao shows a trend toward younger onset, with a median age at diagnosis of 51, which is 8–10 years earlier than in European and Western countries. Among these cases, the proportion of young patients under 40 years old reaches 18.2%. The Macao Special Administrative Region government has identified breast cancer prevention and control as a public health priority.

Since its approval by the FDA in 1998, trastuzumab has been successfully applied in clinical practice and has improved the prognosis of patients with HER2-positive breast cancer. However, the majority of patients who initially respond to trastuzumab-based regimens develop resistance within one year. Furthermore, a significant number of women do not benefit from this therapy at all. According to our hospital-based questionnaire survey in Macao, most female breast cancer patients expressed concerns regarding trastuzumab resistance. Further communication with hospital physicians revealed that trastuzumab resistance significantly impacts their treatment planning for patients, and there is a strong desire for novel therapies that could offer new hope in addressing this resistance. Therefore, it is imperative to develop new treatment strategies to resolve this clinical challenge.
Gemcitabine is a prodrug used in adjuvant and neoadjuvant chemotherapy. It requires cellular uptake and subsequent intracellular phosphorylation to exert its pharmacological activity. The drug exhibits cytotoxic effects by inducing cell death. This is achieved through the incorporation of gemcitabine into elongating DNA strands during DNA synthesis, typically in the S-phase of the cell cycle, which ultimately leads to cell death. One of the cellular metabolites of gemcitabine, dFdCTP, acts as a competitive substrate for deoxycytidine triphosphate (dCTP). This allows dFdCTP to be incorporated into the growing DNA chain. The subsequent addition of deoxyribonucleotides results in the termination of DNA replication, a process known as "masked chain termination" (Mini et al., 2006). In this process, gemcitabine becomes irreversibly incorporated into the DNA. Due to the presence of the terminal nucleotide, proofreading exonucleases are unable to remove the gemcitabine nucleotide from the penultimate position, thereby activating apoptotic pathways in the affected cells. Another characteristic of gemcitabine is its self-potentiating effect, whereby it enhances its own activity. dFdCTP inhibits ribonucleotide reductase (RR), the enzyme responsible for the formation of deoxyribonucleotide triphosphates and the conversion of CDP to dCDP (Jia & Xie, 2015). This leads to a reduction in the intracellular concentration of dCTP required for DNA synthesis. Lower dCTP concentrations diminish its competitive advantage, facilitating the incorporation of dFdCTP (gemcitabine) into the elongating DNA strand. Although conventional gemcitabine chemotherapy initially produces positive therapeutic outcomes, prolonged administration often leads to the development of drug resistance, resulting in gemcitabine-resistant cancer cells. This resistance arises through various mechanisms, including molecular and cellular adaptations such as inactivation of cell death pathways, increased expression of drug efflux pumps, activation of oncogenic signaling pathways, and alterations in mRNA regulation. One such resistance mechanism involves the upregulation of human epidermal growth factor receptor 2 (HER2). In gemcitabine-resistant pancreatic cancer cells, increased HER2 expression and activation are frequently observed. Overexpression of the HER2 receptor triggers downstream signaling pathways that promote cell survival, proliferation, and metastasis, effectively bypassing the cytotoxic effects of gemcitabine. Our survey findings indicate that public awareness regarding the severity of gemcitabine resistance remains insufficient. In contrast, clinical oncologists frequently express concern over this challenging issue of drug resistance.
Thus, resistance to trastuzumab and gemcitabine in tumor cells is not only a medical challenge, but also a pressing public health issue that urgently calls for innovative therapeutic strategies and systematic solutions.
Furthermore, survey results indicate that the general public possesses a preliminary understanding of cancer and chemotherapy, and there is a strong and urgent hope for breakthrough progress in addressing triple-negative breast cancer and cancer drug resistance. Concurrently, questionnaires distributed to frontline clinical physicians reveal that resistance to both gemcitabine and trastuzumab frequently occurs in clinical practice, often posing significant challenges to the implementation of treatment plans. Among drug resistance issues, triple-negative breast cancer presents the most severe scenario, with extremely low cure rates, and hospitals currently lack effective treatment measures against such resistance. We aim to develop a novel therapeutic strategy that can effectively improve the clinical management of trastuzumab and gemcitabine resistance in cancer treatment, while also exploring its potential utility in treating triple-negative breast cancer.
During our competition preparation, we were honored to interview Dr. Yuhao Fu, an expert in the field of synthetic biology. After gaining a deep understanding of our research objectives, Dr. Fu provided highly instructive suggestions: "I recommend that you attempt to design and synthesize a bispecific antibody to address the current therapeutic challenges." Furthermore, he advised us to fully utilize computer-aided design tools to optimize the antibody development process. These expert recommendations provided clear direction for our research and helped us better define the technical pathway of integrating synthetic biology methods with computer-aided design.
In designing our bispecific antibody, the initial step involved identifying two specific targets. Through an extensive review of relevant literature, we identified CD47 and HER2 as suitable target candidates. HER2, being a well-established therapeutic target, is highly expressed in the majority of patients with drug-resistant cancers, offering a viable opportunity for targeted therapy. Meanwhile, CD47 is overexpressed in approximately 70% of cases, where it binds to its receptor SIRPα and delivers a "don't eat me" signal, enabling tumor cells to evade immune clearance. Moreover, these two targets exhibit synergistic effects: inhibiting HER2 directly blocks tumor proliferation signaling pathways, while blocking CD47 relieves immunosuppression and activates macrophage-mediated phagocytosis, resulting in a combined antitumor effect greater than the sum of its parts ("1+1>2"). Therefore, selecting CD47 and HER2 as dual targets for the treatment of trastuzumab-resistant and gemcitabine-resistant cancers is supported by solid scientific rationale and significant clinical value. We have also further explored the potential efficacy of this design in treating triple-negative breast cancer with low HER2 expression. Preclinical studies have reported that bispecific antibodies can significantly enhance antitumor immune responses, achieving a complete tumor regression rate of 42% in animal models and effectively preventing tumor metastasis. Furthermore, in both trastuzumab-resistant and gemcitabine-resistant breast cancer cell lines, CD47 and HER2 expression is significantly upregulated, providing a novel rationale for combined immunotherapy. By simultaneously targeting tumor cells and the immune microenvironment, the CD47/HER2 dual-targeting approach holds great promise for overcoming resistance to conventional therapies. Thus, we ultimately selected CD47/HER2 as our target of interest.
Subsequently, we began attempting to utilize computational methods to assist in designing the bispecific antibody structure. However, we encountered numerous challenging issues during practical implementation. As a result, we consulted Dr. Yang Chen from the Chinese Academy of Sciences, who provided valuable advice on computer-aided design challenges. He recommended that we combine structure-based design of active antibody fragments with traditional virtual screening approaches to rapidly enrich and screen for functional antibody fragments. Finally, he also suggested employing molecular dynamics simulations and binding free energy calculations to computationally evaluate the activity of the selected and designed antibody fragments.
In summary, the harm caused by drug resistance in cancer patients is reflected not only in poor clinical prognosis, but also in increased treatment difficulty and significant socio-economic burden. Integrating background research, survey results, and recommendations from relevant experts, our team has ultimately decided to develop a novel bispecific antibody. This endeavor aims to provide a new therapeutic strategy for addressing trastuzumab resistance, offer a rational combination therapy approach to gemcitabine resistance, and explore potential treatment options for the highly challenging triple-negative breast cancer.
Overview of NanosphinX
Driven by a profound commitment to addressing the critical challenge of drug resistance in cancer therapy—particularly resistance to commonly used agents such as trastuzumab and gemcitabine, which significantly impact patient outcomes—our team developed NanosphinX, a novel CD47/HER2 bispecific antibody. This innovative therapeutic aims to overcome the dual barriers of "immune escape and proliferation signaling" mediated by concurrent overexpression of CD47 and HER2 in resistant tumor cells. By simultaneously blocking both targets, NanosphinX not only inhibits tumor proliferation but also reactivates macrophage-mediated phagocytosis, offering a synergistic mechanism to combat resistant cancers.

Our ultimate goal is to develop a novel strategy capable of reversing immune suppression and inhibiting tumor proliferation, thereby significantly improving survival rates for patients with trastuzumab-resistant breast cancer and gemcitabine-resistant tumors, and offering them new hope for treatment. We are committed to optimizing patient outcomes and striving to make a substantial impact in overcoming cancer drug resistance. We aspire to bring new hope to patients and families affected by this challenging disease, with the potential to ultimately transform the treatment landscape for refractory cancers.
Trastuzumab
Trastuzumab is a recombinant humanized monoclonal antibody that targets human epidermal growth factor receptor 2 (HER2). Since its FDA approval in 1998, trastuzumab has been successfully applied in clinical practice and has significantly improved the prognosis of patients with HER2-positive breast cancer. Trastuzumab exerts its antitumor effects through the following mechanisms:
- Direct inhibition of the HER2 signaling pathway: By binding to the extracellular domain of the overexpressed HER2 receptor on tumor cells, trastuzumab prevents HER2 from forming homodimers or heterodimers with other receptors (such as HER3), thereby suppressing the activation of downstream signaling pathways including PI3K/AKT and MAPK. These pathways play key roles in promoting cell proliferation, survival, and angiogenesis.
- Antibody-dependent cellular cytotoxicity (ADCC): After binding to HER2 on tumor cells, the Fc portion of trastuzumab interacts with Fc receptors on immune cells—such as natural killer (NK) cells—inducing ADCC and leading to direct cancer cell killing.
- Internalization and degradation: Binding to HER2 promotes receptor internalization and degradation, reducing the expression level of HER2 on the cell surface.
Despite the remarkable success of trastuzumab in cancer treatment, the development of primary or secondary resistance remains a major challenge. Most patients who initially respond to trastuzumab-based regimens develop resistance within one year. Consequently, a considerable number of women do not benefit from this therapy at all.
Studies have shown that despite the development of resistance to trastuzumab, HER2 remains highly expressed in resistant cells, indicating that the HER2 signaling pathway continues to be a key driver of tumor proliferation and survival even in the resistant state. Furthermore, compared to non-resistant cells, trastuzumab-resistant tumor cells also exhibit increased expression of CD47—a "don't eat me" signal on the tumor cell surface. By binding to SIRPα on macrophages, CD47 inhibits phagocytic function, enabling immune escape and further contributing to trastuzumab resistance.
To address this dual-barrier mechanism of resistance—where cancer cells upregulate both HER2 (proliferation signal) and CD47 (immune escape signal), forming a combined "immune escape–proliferation signaling" barrier that evades both direct trastuzumab killing and immune-mediated ADCC—targeting both CD47 and HER2 with a bispecific antibody represents a promising novel therapeutic strategy for overcoming trastuzumab resistance.

The trastuzumab (blue) antibody binds to HER2 (green). The cell membrane is indicated in gray.
Gemcitabine
Gemcitabine is a prodrug commonly used in adjuvant and neoadjuvant chemotherapy. It requires cellular uptake and sequential phosphorylation to become activated, exerting its cytotoxic effects primarily by inducing apoptosis. A metabolite of gemcitabine acts as a competitive substrate for deoxycytidine triphosphate (dCTP). It becomes incorporated into elongating DNA strands during synthesis (S-phase), and after the addition of one more nucleotide, replication terminates—a phenomenon known as "masked chain termination." Since proofreading exonucleases cannot remove the gemcitabine nucleotide, the apoptotic pathway is ultimately activated. Gemcitabine also exhibits self-potentiating properties: its metabolites inhibit ribonucleotide reductase (RR), thereby reducing the concentration of dCTP required for DNA synthesis. This facilitates the incorporation of gemcitabine (as dFdCTP) into DNA, enhancing its efficacy. Although gemcitabine shows significant initial therapeutic effects, long-term use often leads to drug resistance.
The mechanisms of resistance are complex and include apoptosis inactivation, increased drug efflux, and activation of oncogenic signaling pathways. One key mechanism involves the upregulation and activation of human epidermal growth factor receptor 2 (HER2). In resistant pancreatic cancer cells, HER2 overexpression triggers downstream signaling pathways that promote cell survival, proliferation, and metastasis. It activates anti-apoptotic proteins and enhances DNA repair, effectively bypassing the toxicity of gemcitabine. Therefore, combining gemcitabine with HER2-targeted agents represents a promising strategy to overcome resistance and improve treatment outcomes. Further investigations have revealed that CD47 is also highly expressed alongside HER2 on the surface of gemcitabine-resistant tumor cells. This suggests that tumor cells may exploit this dual overexpression to evade immune surveillance and further enhance drug resistance. Consequently, designing a bispecific antibody targeting both CD47 and HER2 offers a potential innovative therapeutic approach to address gemcitabine resistance.
Solution
Driven by a profound commitment to addressing the challenging issue of drug resistance in cancer treatment and exploring novel therapeutic strategies for cancer patients—particularly resistance to the most widely used biological macromolecular drug trastuzumab and the chemotherapeutic agent gemcitabine—we recognize this as an urgent and critical challenge that severely impacts patient prognosis and treatment efficacy. Through in-depth research and data analysis, we discovered a common mechanism in trastuzumab-resistant breast cancer cells and gemcitabine-resistant tumor cells: they consistently overexpress both CD47 and HER2 targets, thereby forming a "dual barrier of immune escape and proliferation signaling," which effectively enables escape from the killing effects of conventional drugs.
To tackle this challenge, we have innovatively designed and developed the CD47/HER2 bispecific antibody, NanosphinX. This bispecific antibody employs a synergistic strategy that simultaneously targets and blocks these two critical targets: one arm counteracts CD47-mediated immune suppression, significantly enhancing macrophage phagocytosis (ADCP); the other arm inhibits the HER2 signaling pathway, directly suppressing tumor proliferation. Our team hypothesizes that this dual blockade can effectively disrupt the defense mechanisms of resistant cells and generate a synergistic antitumor effect surpassing that of single-target approaches.

Our ultimate goal is to significantly improve the survival rates of patients with trastuzumab-resistant tumors through this groundbreaking bispecific antibody strategy, while also exploring novel combination therapies involving gemcitabine and investigating its efficacy against refractory cancers such as triple-negative breast cancer. We aim to bring new therapeutic hope to patients and families facing these severe challenges. We are committed to establishing a novel treatment paradigm to overcome cancer drug resistance, ultimately transforming the landscape of therapy for resistant tumors and prolonging the survival of patients with resistant cancers.
Approach
Our project employs an innovative bispecific antibody design approach to develop a novel active antibody, NanosphinX, for the treatment of trastuzumab-resistant breast cancer cells or gemcitabine-resistant tumor cells. This strategy aims to simultaneously target two molecular targets that are highly expressed in resistant tumor cells, achieving a synergistic therapeutic effect ("1+1>2") while reducing the hematological toxicity associated with the CD47-targeting moiety, thereby ensuring drug safety. NanosphinX is designed with two distinct targeting moieties: one directed against CD47 and the other against HER2. These receptors are frequently overexpressed in trastuzumab-resistant and gemcitabine-resistant tumor cells and play crucial roles in tumor growth, survival, and drug resistance. By targeting both HER2 and CD47, NanosphinX inhibits the signaling functions of these receptors. This dual inhibition is expected not only to slow the proliferation of resistant cells but also to potentially resensitize them to gemcitabine and trastuzumab.
To achieve this goal, we designed and synthesized a vector carrying the full-length coding sequence. We employed a plasmid vector into which the designed sequence was inserted at the multiple cloning site. This was followed by transformation to introduce the recombinant plasmid into E. coli (strain BL21). After successful transformation, the bacteria were cultured to amplify the plasmid, which was then extracted using the Tiangen Plasmid Maxi Prep Kit. Finally, following the instructions provided in the ExpiCHO™ Expression System User Guide, the plasmid was transiently transfected into CHO cells for antibody expression. Once synthesized, the resulting bispecific nanobody was purified and tested using in vitro cell-based assays. Its synthesis was validated via sequencing, and its efficacy was further examined.

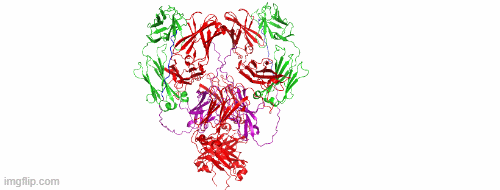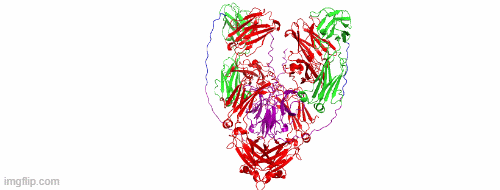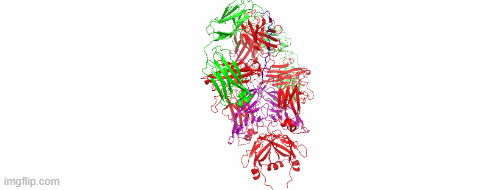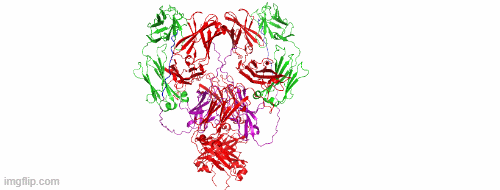

CD47/HER2
Our dry lab genomic bioinformatics analysis, utilizing the GTEx and TCGA databases, revealed a significant upregulation of HER2 and CD47 mRNA expression in drug-resistant tumor cells (with p-values of 5.12×10⁻³⁵ and 8.76×10⁻¹⁸, respectively).
HER2 (human epidermal growth factor receptor 2) is a cell surface tyrosine kinase associated with cell growth and division. CD47 is an important cell surface protein that functions as a "don't eat me" signal by binding to SIRPα on macrophages, enabling cancer cells to evade phagocytosis by the immune system. Both proteins consist of three major structural domains: an extracellular region that binds specific molecules, a transmembrane segment, and an intracellular region. When activated, HER2 stimulates cell growth and division, while CD47 promotes tumor immune escape by inhibiting phagocytosis.
Our further analysis revealed that not only were these genes overexpressed, but they also independently predicted poorer overall survival in cancer patients (HER2: p = 0.0035; CD47: p = 0.018). Notably, high expression levels of HER2 and/or CD47 were significantly associated with reduced patient survival (p = 0.00056). These findings suggest that HER2 and CD47 represent promising therapeutic targets for improving outcomes in cancer patients.
To further investigate the association between these two genes and tumor immune escape as well as therapy resistance, we analyzed a database of pancreatic cancer cell lines (e.g., Panc-1 and MDA-MB-231) known for their high immune escape potential and resistance to conventional therapies. Our findings demonstrated that both HER2 and CD47 were significantly overexpressed in these resistant and highly immune-evasive tumor cells. To determine the clinical relevance of this observation, we conducted a retrospective analysis of 85 patients who had received standard-of-care treatment. Consistent with the cell line data, we observed that HER2 and/or CD47 were more frequently expressed in cancer patients exhibiting immune escape and treatment resistance compared to those responsive to therapy (p = 0.03).
Based on these preliminary data, we hypothesized that simultaneously targeting HER2 and CD47 would not only inhibit tumor cell proliferation but also disrupt their immune evasion mechanisms, thereby enhancing the immune system's antitumor activity. Through wet lab experiments, we confirmed that NanosphinX, by concurrently targeting HER2 and CD47, significantly suppressed tumor cell growth, enhanced macrophage-mediated phagocytosis of cancer cells, and exhibited strong efficacy against resistant tumor cells.

Overall, this dual-targeting strategy holds promise in addressing the challenges posed by inadequate immune and targeted therapies as well as chemotherapy resistance, offering new therapeutic breakthroughs for patients with resistant breast cancer.
References
- Dheilly E, Majocchi S, Moine V, Didelot G, Broyer L, Calloud S, Malinge P, Chatel L, Ferlin WG, Kosco-Vilbois MH, Fischer N, Masternak K. Tumor-Directed Blockade of CD47 with Bispecific Antibodies Induces Adaptive Antitumor Immunity. Antibodies (Basel). 2018 Jan 3;7(1):3. doi: 10.3390/antib7010003. PMID: 31544856; PMCID: PMC6698848.
- Haopeng Rui, Xiaofeng Yang, Jiahao Chen, Jia Wang, Zhiqiang Zheng, Zhiqiang Chen, Jingtao Lu, Peggy Wu, Janet Chen, Allison Wang, George Chen; Abstract 1873: D3L-001, a novel bispecific antibody targeting HER2 and CD47, demonstrates potent preclinical efficacy in solid tumors. Cancer Res 1 April 2023; 83 (7_Supplement): 1873. https://doi.org/10.1158/1538-7445.AM2023-1873
- Advani, R., Flinn, I., Popplewell, L., Forero, A., Bartlett, N.L., Ghosh, N., Kline, J., Roschewski, M., LaCasce, A., Collins, G.P., Tran, T., Lynn, J., Chen, J.Y., Volkmer, J.- P., Agoram, B., Huang, J., Majeti, R., Weissman, I.L., Takimoto, C.H., Chao, M.P., Smith, S.M., 2018a. CD47 blockade by Hu5F9-G4 and rituximab in Non-Hodgkin's lymphoma. N. Engl. J. Med. 379, 1711–1721. https://doi.org/10.1056/ nejmoa1807315.
- Liu, M., O'Connor, R.S., Trefely, S., Graham, K., Snyder, N.W., Beatty, G.L., 2019. Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47− mediated 'don't-eat-me' signal. Nat. Immunol. 20, 265–275. https://doi.org/10.1038/s41590-018-0292-y
- Zhang B, Shi J, Shi X, Xu X, Gao L, Li S, Liu M, Gao M, Jin S, Zhou J, Fan D, Wang F, Ji Z, Bian Z, Song Y, Tian W, Zheng Y, Xu L, Li W. Development and evaluation of a human CD47/HER2 bispecific antibody for Trastuzumab-resistant breast cancer immunotherapy. Drug Resist Updat. 2024 May;74:101068. doi: 10.1016/j.drup.2024.101068. Epub 2024 Feb 13. PMID: 38402670.