
Overview

The Challenge
The H5N1 avian influenza pandemic threatens global food security, with poultry diseases costing ~20% of gross production value—even higher in developing countries. Vaccine inequity is stark: in a crisis, <2% of influenza vaccine supply would reach low- and middle-income countries, and weak cold-chain infrastructure further compromises vaccine efficacy.
Protection Gap
Traditional vaccines require weeks to induce protective immunity—leaving birds exposed at outbreak onset
Strain Variability
Continuous viral evolution (H5N1) undermines reliance on strain-matched formulations for future waves
Cold-Chain Dependency
Weak infrastructure in developing regions compromises vaccine efficacy and distribution
Economic Barriers
Small-scale farmers cannot afford current programs or trained personnel for injectable vaccines
The Solution:
This convergence demands a fundamentally different approach: a thermostable, orally administered, affordable platform that delivers both instant (passive) and long-term (active) immunity and is deployable in resource-limited settings.
Active vs. Passive Immunity
Arises when the host's immune system is stimulated by antigen (e.g., vaccination), generating de novo antibodies and cellular responses and establishing immunological memory.
Provided by preformed antibodies (e.g., purified antibodies, nanobodies, immune sera) that confer immediate protection. Does not generate memory and is therefore transient.
🔗 Integrated Platform
An integrated platform that pairs passive (instant) with active (long-lasting) immunity can cover both the early outbreak gap and sustained protection, especially in settings where rapid deployment and affordability are essential.
Engineering Approach
AvianGuard is a dual-immunization platform that stands out because of its:
Modular Architecture
Plug-and-play design for rapid adaptation
Probiotic Compatibility
Oral delivery for ease of administration
Self-Assembly
Post-translational processing without extra steps
Field Robustness
Stable under real-world conditions
However, these features come from several iterations of the DBTL cycle. Below we detail our journey focusing on the passive immunity component.
DBTL Cycle 1

Our initial chassis was Lactococcus lactis for oral delivery. We designed a constitutive expression cassette driven by the strong CP44 promoter to produce a bispecific nanobody arranged in tandem (NB10–linker–R1A5), preceded by the Usp45 signal peptide to target secretion through the Sec pathway. The design avoids competitive antigenic interference when both arms (active + passive) are used in parallel.
Strategic Epitope Selection (non-interfering with active immunity):
- R1a-A5: Binds a region spanning from the fusion peptide to residue 68 in HA2 and shows cross-neutralization against subtype H1.
- Nb10: Binds to a conserved receptor-binding site in the HA1 domain with affinity for the 130-loop, 150-loop, and 220-helix, with strong neutralization against clades 2.3.2.1 and 2.3.4.4 that have been associated with human infections and high fatality rates.
The full ORF (NB10–linker–R1A5) including Usp45 and standard regulatory elements was synthesized, and plasmid cloning/propagation was attempted in E. coli cloning strains. Vendors (Twist and Ansa) reported that although the sequence itself was readily synthesizable, E. coli transformants carrying the constitutive cassette could neither be cloned nor propagated.
Linear Schema of the Passive Circuit (TU):
- CP44: strong constitutive promoter
- RBS: optimized ribosome binding site
- Usp45: signal peptide (Sec pathway)
- NB10 — Linker — R1A5: bispecific tandem nanobody against Hemagglutinin of avian influenza
Mechanistically, a strong constitutive promoter sustains high transcription in the absence of induction. For a secretory tandem protein, this creates substantial metabolic load and membrane/periplasmic stress, compromising the viability of E. coli hosts that acquire the plasmid.
Although Usp45 originates from L. lactis, its Sec-type signal can be recognized by the E. coli Sec machinery, routing the fusion toward the periplasm; export efficiency is context-dependent and not all heterologous precursors are exported efficiently.
When substrate load is high or processing is incomplete, export intermediates accumulate at the membrane/periplasm and trigger envelope-stress responses such as Cpx and Psp, further depressing growth. The NB10–linker–R1A5 tandem also increases the burden on Sec and raises the risk of misfolding/aggregation in the periplasm.
CONCLUSION
The concurrence of constitutive expression, Usp45-mediated periplasmic trafficking, and the tandem nanobody architecture explains the cloning failures in E. coli under a constitutive scheme, despite the sequence being perfectly synthesizable.
Takeaway: Temporal control of expression—i.e., switching to an inducible system—reduces Sec load during cloning/propagation and helps prevent early cell death, making constitutive expression unsuitable for this cassette at the cloning stage.
DBTL Cycle 2
To make the bispecific nanobody robust for field use (delivery in drinking water or feed; variable pH; environmental proteases), we proposed intein-mediated head-to-tail (N–C) cyclization of NB10–linker–R1A5 to:
- Increase protease resistance
- Improve thermal/overall stability while preserving affinity in complex matrices
Inteins (Quick Primer)
Inteins are self-excising protein elements that catalyze protein splicing, ligating the flanking exteins with a native peptide bond. They occur as cis inteins (single polypeptide) or split inteins (separate N-intein and C-intein that associate in trans). The canonical pathway proceeds via acyl shifts, transesterification, and Asn cyclization, ending with an S→N acyl shift that seals the peptide bond.
Npu DnaE split intein is widely used for cyclization because it is fast, efficient, and relatively permissive across conditions.
Chosen System
Split intein Npu-DnaE from Nostoc punctiforme (N- and C-parts) rejoin and excise themselves; placing them in C-intein—peptide—N-intein order yields a head-to-tail cyclic peptide. Cyclization typically improves stability (less enzymatic degradation, better thermal tolerance, extended in-host half-life).
We also planned extein optimization using CWN (+1/+2/+3) and GGH (−3/−2/−1) at splice junctions to accelerate splicing.
In-silico Construct Assembly
Under a controllable promoter; codon usage and RBS tuned for the intended chassis.
- Modularity: keep NB10/R1A5 in swappable cassettes (defined restriction sites) to facilitate alternative binders.
- Splicing context: specify linker lengths (G₄S repeats) around the splice junctions to reduce steric hindrance and promote folding/splicing.
- Analytical plan drafted: (no lab execution): predicted splicing kinetics, structure/solubility checks, and secretion routing scenarios.
(Planned; literature-driven risk assessment) Guided by prior reports and protein-biogenesis principles, we anticipated low efficiency and poor reproducibility when coupling intein-mediated cyclization to Usp45-dependent secretion in Lactococcus lactis (Gram+).
In Gram-positives, secretion delivers nascent chains directly to the cell-wall/extracellular space, a microenvironment with non-physiological redox potential, variable pH, and abundant surface proteases. These conditions are unfavorable for intein catalysis and can destabilize or clip splicing intermediates, yielding incomplete or heterogeneous cyclization.
Given the conceptual/literature basis and the risks above, we did not implement cyclization in L. lactis. Instead, we decided to migrate to a chassis that enables controlled intracellular (and/or periplasmic) cyclization prior to any export—the probiotic E. coli Nissle 1917 under pLux (AHL) control.
This preserves the stability gains of cyclization while minimizing conflicts with secretion/anchoring pathways.
DBTL Cycle 3

We selected E. coli Nissle 1917 (EcN) under the pLux (AHL) promoter to:
- Express the NB10–linker–R1A5 construct and achieve intein-driven cyclization before any export (i.e., completing splicing intracellularly and/or in the periplasm as needed)
- Control expression stringently—minimizing leak during cloning and enabling AHL-induced production
- Tune the microenvironment (pH, ions, temperature/time) to favor proper folding and cyclization
To obtain a desired promoter range (lower expression than the previous existing device BBa_K3893028), we started using the mathematical model derived from the characterization of that part, and then we performed a parametric simulation introducing a wildcard RBS, represented by a variable translation rate (pR).
The model is:
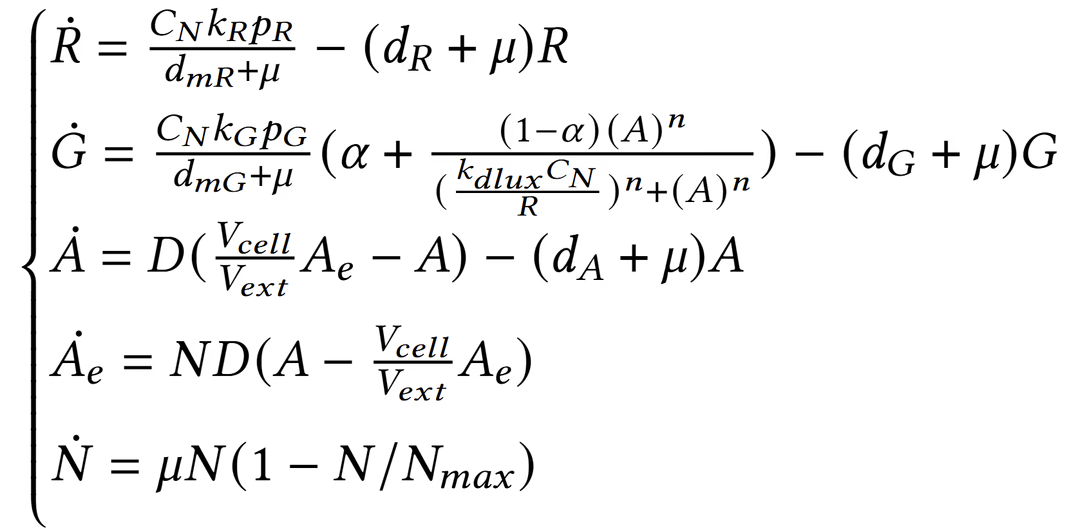
We explored a range of RBS strengths computationally, keeping all other parameters constant with the values from the DBTL characterization.
| Parameter | Description | Value | Units |
|---|---|---|---|
| k_G | Transcription rate | 9.1317 | min⁻¹ |
| kdlux | LuxR–AHL to promoter dissociation constant | 71549 | molecules |
| alpha | Basal expression | 0.050 | adim |
| n | Hill coefficient | 3.2382 | adim |
| CN | Plasmid copy number | 30 | plasmids |
| dmg | mRNA degradation rate | 0.2335 | min⁻¹ |
| dg | Protein degradation rate | 0.0040 | min⁻¹ |
For this Design, we proposed a pR = 0.5 (relative scale) as the target translation efficiency for the new construct.
Simulations predicted that this would yield a lower GFP steady-state level while maintaining the same activation range as the original design.
After comparing the model output with the relative translation efficiencies of standard iGEM RBSs, we selected BBa_B0032 as the best match to achieve the desired behavior.

We constructed and characterized the new device BBa_253I2NAK following the same methodology as in the first iteration. E. coli 10G was transformed with the plasmid, grown overnight in LB medium with antibiotics, and normalized before measurement. Samples (200 µL) were distributed into 96-well plates and incubated at 37 °C and 230 rpm under double orbital shaking.
Fluorescence (excitation 488 nm, emission 530 nm) and OD₆₀₀ were recorded every 5 minutes for 20 hours, with ten AHL concentrations (0–500 µM) tested in triplicate. Calibration followed the InterLab 2023 protocols, ensuring reproducibility.
Experimental data confirmed a lower fluorescence response compared to the high-expression device, matching the predicted range. However, a fine re-optimization is necessary in order to really capture the behaviour.

The experimental results were compared to the model predictions without re-optimizing parameters. The model accurately reproduced the new fluorescence data, validating that the behavior of BBa_253I2NAK could be quantitatively predicted from the parameters obtained in the first iteration. To finish the Learn stage, we performed a re-optimization of only the pR parameter corresponding to the RBS.
The parameter estimation was performed by minimizing the following cost function:
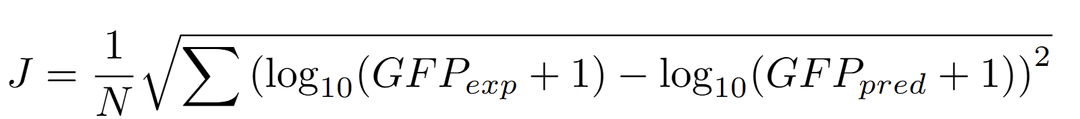
This objective function measures the root mean squared error between the experimental and simulated (predicted) GFP fluorescence levels in log scale, ensuring balanced weighting across induction ranges.
The optimization was carried out using the Bayesian Adaptive Direct Search (BADS) algorithm, which efficiently explores the parameter space to locate global optima. The process yielded a value for pR = 0.82853 min^-1 (corresponding to the RBS BBa_B0032). The corresponding predicted response after the re-optimization (LEARN), together with the original prediction at the beginning of the iteration (DESIGN) and the experimental results (TEST):

Moreover, we performed a dynamic simulation (how the expression changes in time after the induction) and compared it with the time-course experimental data calibrated after induction with three AHL inductions: no inducer (0 nM), middle (75nM), and high inducer (300nM). The line is the model prediction and the shadow area is the mean and standard deviation of the experimental data. The next plot shows the strong agreement, especially for long times aproaching the steady state.

This strong agreement between model and data confirms the transferability of the pLux promoter model and demonstrates the predictive power of the DBTL cycle.
Final Design
We engineered a passive-immunity platform that expresses and secretes cyclized nanobodies ("cyclobodies") using a split-intein cassette under pLux/LuxR–AHL control.
The construct performs post-translational self-assembly, yielding a head-to-tail cyclic, bispecific nanobody that is more resistant to proteolysis and heat—well suited for field deployment in water/feed matrices.
Strategic restriction sites flank Nano-A and Nano-B, allowing rapid swap-in of alternative binders for other targets or viral families.
Final Construct Architecture:

Key Advantages:
References
Bhattacharyya, S., Bershtein, S., Yan, J., Argun, T., Gilson, A. I., Trauger, S. A., & Shakhnovich, E. I. (2016). Transient protein–protein interactions perturb E. coli metabolomics and cause gene dosage toxicity. PLOS Genetics, 12(12), e1006235.
Boada, Y., Vignoni, A., Alarcon‐Ruiz, I., Andreu‐Vilarroig, C., Monfort‐Llorens, R., Requena, A., & Picó, J. (2019). Characterization of gene circuit parts based on multiobjective optimization by using standard calibrated measurements. ChemBioChem, 20(20), 2653-2665.
Cheriyan, M., Pedamallu, C. S., Tori, K., & Perler, F. (2013). Faster protein splicing with the nostoc punctiforme DnaE intein using non-native extein residues. Journal of Biological Chemistry, 288(9), 6202–6211.
Darwin, A. J. (2005). The phage shock protein response. Molecular Microbiology, 57(3), 621–628.
Ellis, D., et al. (2023). Antigen spacing on protein nanoparticles influences B cell activation. Cell Reports, 42(5), 112521.
Hemmi, S., Asano, R., Kimura, K., Umetsu, M., Nakanishi, T., Kumagai, I., & Makabe, K. (2020). Construction of a circularly connected VHH bispecific antibody (cyclobody) for the desirable positioning of antigen-binding sites. Biochemical and Biophysical Research Communications, 523(1), 72–77.
Jones, S. E., Lloyd, L. J., Tan, K. K., & Buck, M. (2003). Secretion defects that activate the phage shock protein response in Escherichia coli. Journal of Bacteriology, 185(21), 6707–6711.
Karyolaimos, A., Zhang, Z., Su, M., & Hatzimanikatis, V. (2019). Enhancing recombinant protein yields in the E. coli periplasm: current pathways and engineering strategies. Frontiers in Microbiology, 10, 1511.
Lee, H. C., & Bernstein, H. D. (2001). The targeting pathway of Escherichia coli presecretory and membrane proteins. Proceedings of the National Academy of Sciences, 98(7), 3471–3476.
Le Loir, Y., Azevedo, V., Oliveira, S. C., Freitas, D. A., Miyoshi, A., Bermúdez-Humarán, L. G., Nouaille, S., Ribeiro, L., Le Loir, Y., & Langella, P. (2005). Protein secretion in Lactococcus lactis: An efficient way to increase the overall heterologous protein production. Microbial Cell Factories, 4(1), 2.
Le Loir, Y., Gruss, A., Ehrlich, S. D., & Langella, P. (2001). Signal peptide and propeptide optimization for heterologous protein secretion in Lactococcus lactis. Applied and Environmental Microbiology, 67(9), 4119–4127.
Lozano Terol, G., Galán-Vásquez, E., François, J. M., & Tauch, A. (2021). Impact of the expression system on recombinant protein production: balancing burden and productivity. Frontiers in Microbiology, 12, 682001.
Mao, J., Liu, Z., & Shin, J. (2024). Relieving metabolic burden to improve robustness and productivity in engineered microbes. Journal of Biotechnology, 380, 1–12.
Michon, C., Langella, P., Eijsink, V. G. H., Mathiesen, G., & Chatel, J. M. (2016). Display of recombinant proteins at the surface of lactic acid bacteria: Strategies and applications. Microbial Cell Factories, 15, 70.
Plavec, T. V., Štrukelj, B., & Berlec, A. (2019). Screening for new surface anchoring domains for Lactococcus lactis. Frontiers in Microbiology, 10, 1879.
Raivio, T. L. (2013). The Escherichia coli Cpx envelope stress response: linking membrane protein folding to cellular physiology. FEMS Microbiology Letters, 344(2), 189–200.
Snoeck, S., Reumers, J., & Tagkopoulos, I. (2024). "Metabolic burden" explained: Stress symptoms and mechanisms during overexpression in E. coli. Computational and Structural Biotechnology Journal, 22, 6869–6888.
Tavassoli, A. (2017). SICLOPPS cyclic peptide libraries in drug discovery. Current Opinion in Chemical Biology, 38, 30–35.
Tay, P. K. R., Lim, P. Y., & Ow, D. S.-W. (2021). A SH3_5 cell anchoring domain for non-recombinant surface display on lactic acid bacteria. Frontiers in Bioengineering and Biotechnology, 8, 614498.
Truebestein, L., & Leonard, T. A. (2016). Coiled-coils: The long and short of it. BioEssays, 38(9), 903–916.
Valent, Q. A., Kendall, D. A., High, S., Kusters, R., Oudega, B., & Luirink, J. (1998). The E. coli SRP and SecB targeting pathways for inner-membrane proteins. The EMBO Journal, 17(9), 2504–2512.