Overview
Diabetic foot ulcer (DFU) is a chronic wound condition caused by multiple factors, including persistent inflammation, tissue necrosis, and infection. To improve wound healing process, we developed a specialized patch designed for DFU. This patch includes a split intein system to conjugate therapeutic proteins with a collagen-binding domain (CBD), enabling their stable immobilization within the collagen matrix of the patch. This modular approach not only ensures protein retention on the patch but also provides flexibility for simultaneously anchoring multiple therapeutic proteins, addressing the multifactorial challenges of DFU.
AGE-Thwart Patch
Our goal is to create a hydrogel patch for DFU that integrates sRAGE for AGE capture and biocompatible materials for tissue repair. The patch consists of two interconnected systems: a protein system containing sRAGE and CBD, while a material system composed of collagen and chitosan for tissue repair and anti-microbial activities. Together, these systems provide both therapeutic functionalities to disrupt AGE-driven inflammation and the functional support necessary for effective wound healing.
Protein System – Core Therapeutic Protein Design
Engineering sRAGE to Enhance Binding Affinity for AGEs
sRAGE, as a soluble receptor for AGEs, is composed of three domains: V, C1, and C2, with the V domain serving as the binding site for AGEs. (Zhou et al., 2024) To compete the AGE binding with membrane-bound RAGE, we need to enhance the sRAGE binding affinity to AGE. By surveying lectures, we found that N-glycan depletion mutation (N25Q and N81Q) in the V domain could enhance its binding affinity toward AGEs. (Osawa et al., 2007)
CBD-Mediated Immobilization to Stabilize sRAGE on Hydrogel
Embedding sRAGE directly into the hydrogel would cause it to diffuse away too quickly, making sustained AGE removal ineffective. To prevent this, we incorporated a Collagen-Binding Domain (CBD) to stably anchor sRAGE within the patch. This prolongs protein retention and avoids loss of activity from chemical crosslinking. About the CBD sequence, we selected the leucine-rich repeat (LRR) 5–7 region of Lumican, reported to bind collagen with very low Kd values, ensuring strong, non-covalent attachment. (Kalamajski, S., & Oldberg, Å., 2009)
Incorporation of Split Intein to Establish a Modular Platform
To save flexibility for adapting the patch for other
clinical needs in the future, we introduced the Npu DnaE
split intein system. Split intein is a type of intein
system composed of two protein fragments, IntN and IntC.
This system can spontaneously splice two protein fragments
without the need for external cofactors or energy. When
the intein fragments associate to form an active intein,
the intein sequence is excised through electron transfer,
and the two flanking sequences are ligated to generate a
single continuous fusion protein. (Iwai, Hideo, et al.,
2006)
This property enables modular assembly of sRAGE or other
therapeutic proteins (e.g. antimicrobial peptides) with
CBD. In this way, the patch remains flexible, supporting
multiple protein functions while maintaining stable
immobilization.
To make the intein splicing more efficient, we also
optimized the flanking sequences around the intein. The
amino acid AEY to RGK at N terminal, CFN to CWE at C
terminal to maximize splicing efficiency. (Cheriyan, et
al., 2013)
This strategy ensures that sRAGE or other protein remains
immobilized in the hydrogel to effectively through CBD, so
sRAGE can capture AGEs at the wound site, while also
preserving modularity, allowing the patch to be adapted
for other therapeutic proteins in chronic wound treatment.
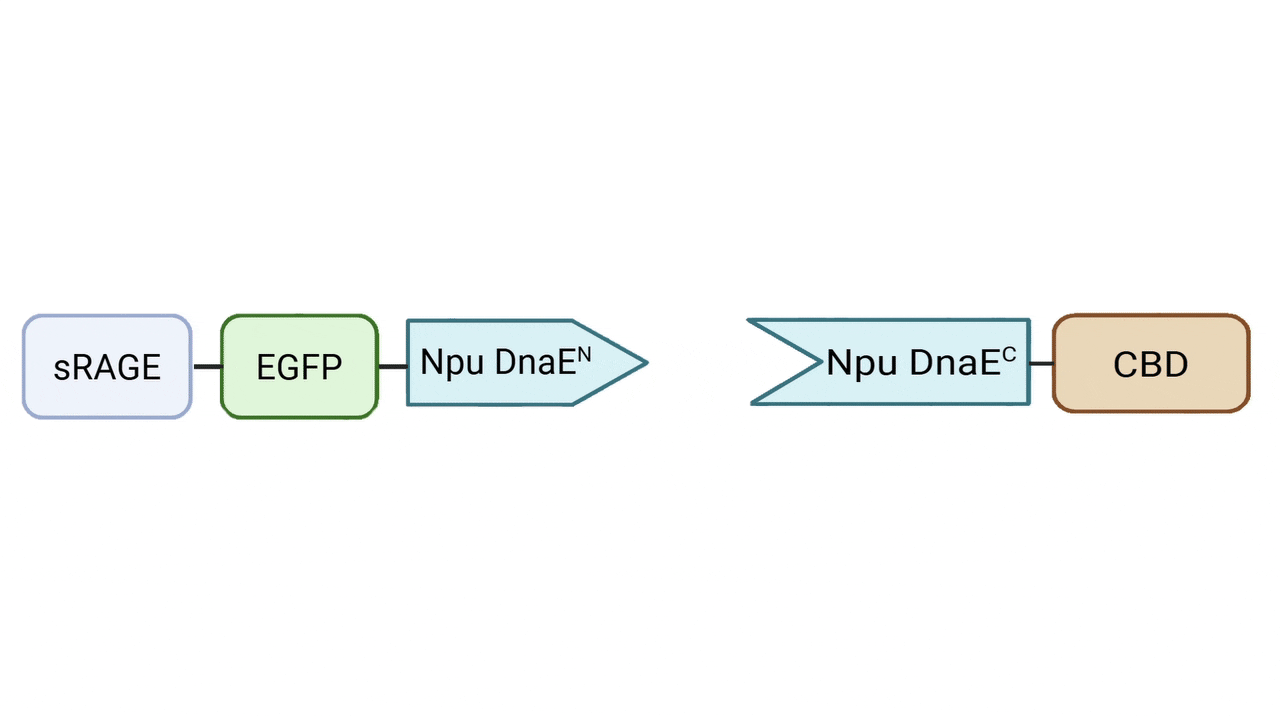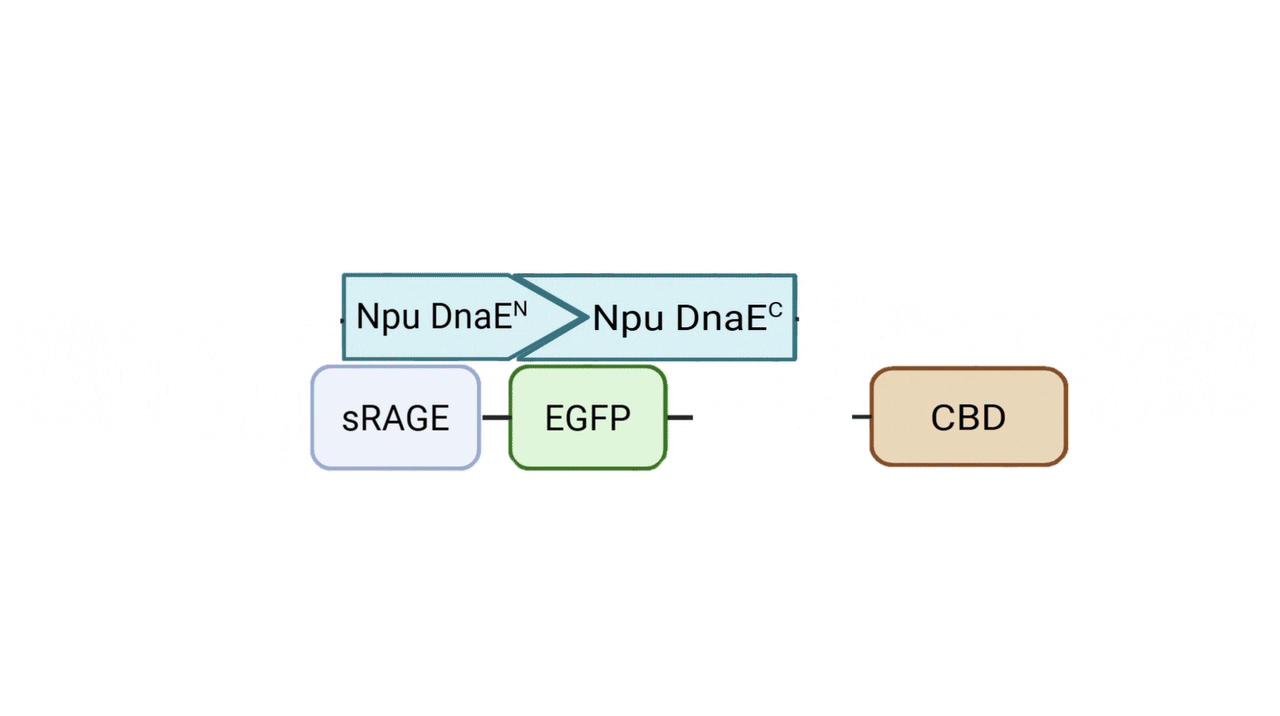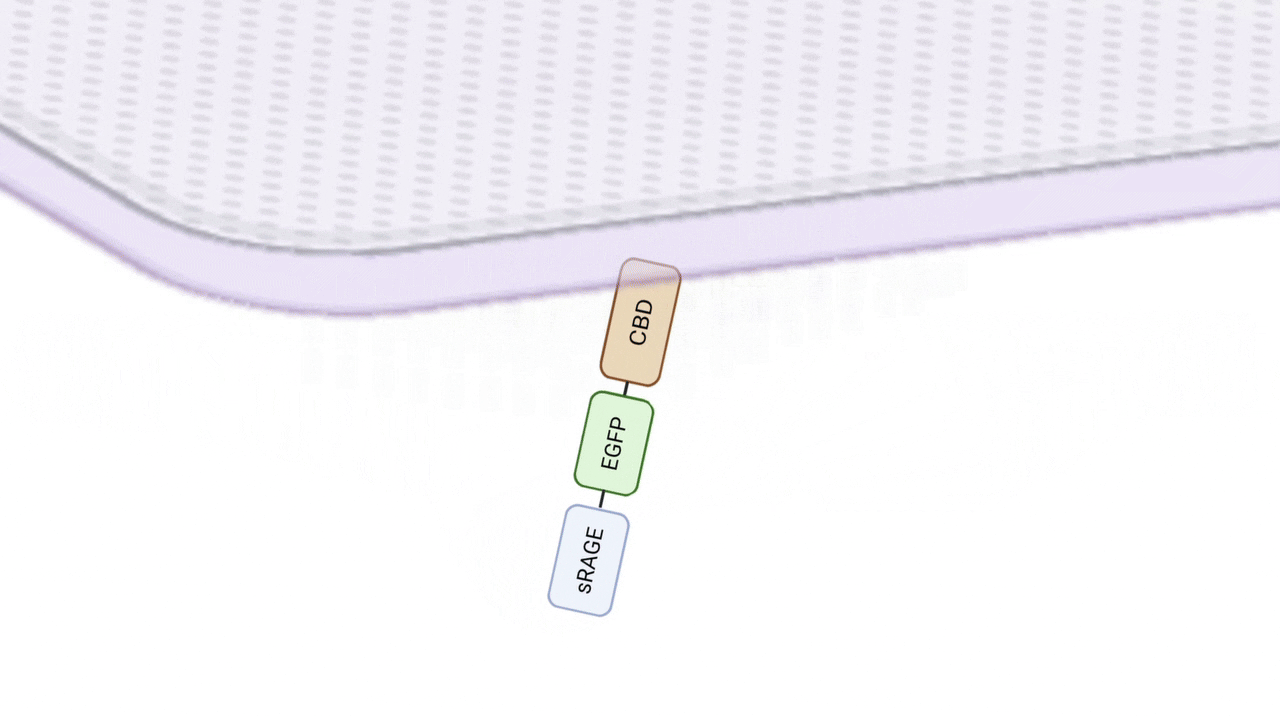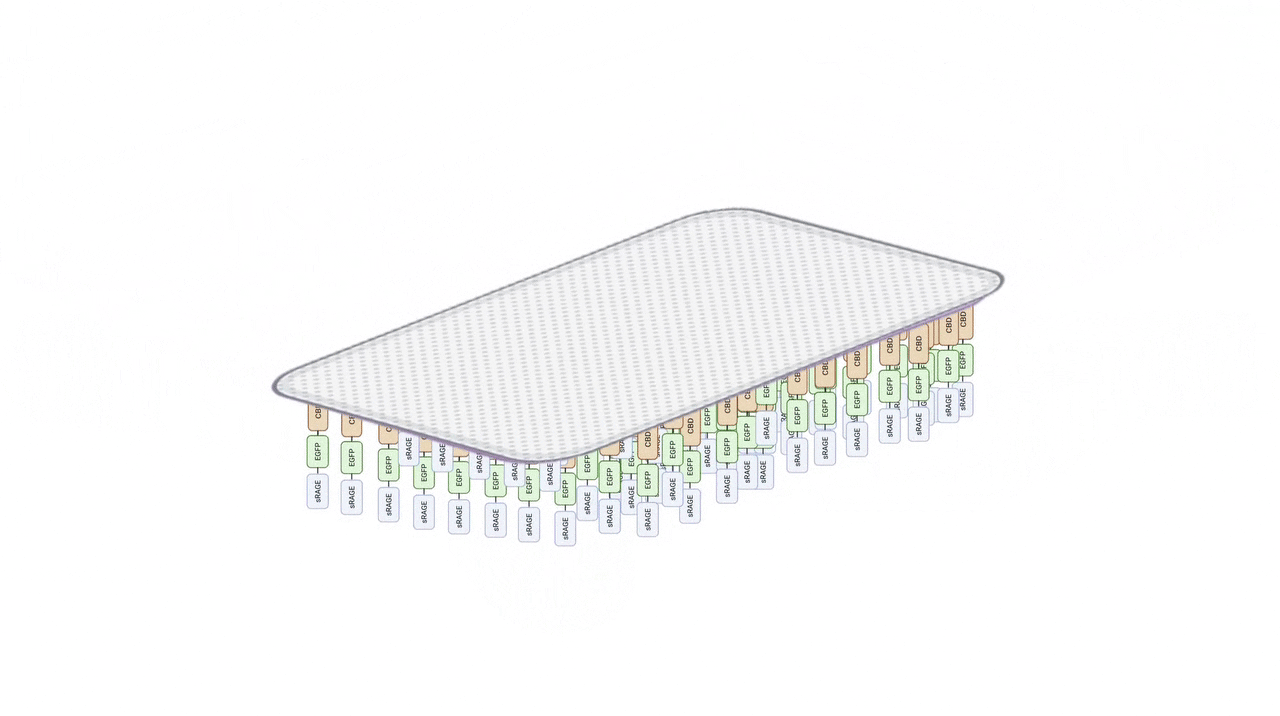
Protein Construct Design
Each fragment was codon optimized for E. coli or HEK293T
expression systems. This design allows for rapid,
cost-effective expression, while retaining the option for
proper folding and secretion in mammalian cells to achieve
better protein expression when necessary. For details
on experimental validation and optimization, see
[Engineering].
N-terminal function Fragment—Signal Peptide–sRAGE/ mut-sRAGE–EGFP–6xHis–RGK–Npu-DnaEN
The N-terminal fragment serves as a functional effector
unit for AGE capture, while retaining flexibility for
future therapeutic substitution. In this version, sRAGE
(or its affinity-enhanced mutant) serves as the primary
effector to mediate AGE binding, while EGFP is included
to monitor localization and stability on the patch.


- Signal peptide: Applying in mammalian expression system to direct the secretion of the interested protein, mimicking native sRAGE production.
- sRAGE: Positioned at the N-terminus of fusion protein to avoid steric hindrance from downstream domains and ensure efficient AGE binding. The sequence was derived from the human sRAGE but lacked the native C-terminal transmembrane domain to prevent membrane anchoring. For the mammalian construct, two N-linked glycosylation sites (N25Q and N81Q) were mutated to assess the effect of de-glycosylation on AGE-binding affinity.
- EGFP: Inserted between sRAGE and Npu-DnaEN to serve as a fluorescent reporter, allowing visualization of protein localization and stability on hydrogel patches.
- RGK (flanking sequence): Designed based on the native extein residues of the Npu-DnaE intein system and positioned next to the intein junction to promote efficient trans-splicing.
- Npu-DnaEN: The N-terminal split intein domain, located at the C-terminus of the N-terminal function fragment , enabling covalent ligation with C-terminal anchorage fragment.
- 6xHis tag: Facilitates affinity purification and detection.
C-terminal anchorage Fragment— 6xHis–Npu-DnaEC–CWE–CBD / Npu-DnaEC–CWE–6xHis–CBD
The C-terminal fragment mediates stable anchorage of the fusion protein onto the hydrogel patch and incorporates a collagen-binding domain (CBD) for site-specific immobilization.

- 6xHis tag: Provides an additional purification handle. we try the 6xHis tag locate in front of C-half intein or CBD to gain the best one for protein purified and intein splicing.
- Npu-DnaEC: The C-terminal split intein domain, which pairs with Npu-DnaEN for intein-mediated ligation with N-terminal function fragment.
- CWE (flanking sequence): Designed based on the native extein residues of the Npu-DnaE intein system and positioned adjacent to the intein junction to promote efficient trans-splicing.
- CBD (collagen-binding domain): Enables site-specific immobilization onto collagen-based hydrogel scaffolds.
The splicing reaction generates a native peptide bond between N and C-terminal fragments, covalently linking sRAGE to CBD. With two N-terminal and two C-terminal variants constructed, a total of four fusion combinations can be produced for comparative evaluation.
Material System – Hydrogel Patch Structure
Initial wounds in diabetic foot patients are often accompanied by chronic
inflammation, delayed healing due to poor circulation, decreased immune
function, and the accumulation of AGEs. To effectively promote wound healing,
we aimed for a material that combines antibacterial, moisturizing, and
tissue-regenerative properties. To achieve this, we designed the patch with
a bilayer patch.
Medical-Grade PU Film: Barrier Protection and Moisture Retention for Fragile Skin
The outer layer is composed of a medical-grade polyurethane (PU) film, which provides reliable physical protection by blocking external contaminants to reduce the risk of infection. PU film is both breathable and capable of preventing hydrogel dehydration, thus maintaining a moist environment. Since ulcerated skin is fragile, the PU film’s softness, transparency, and high conformity make it easy to handle in clinical use, while minimizing the risk of secondary injury to the patient’s skin.

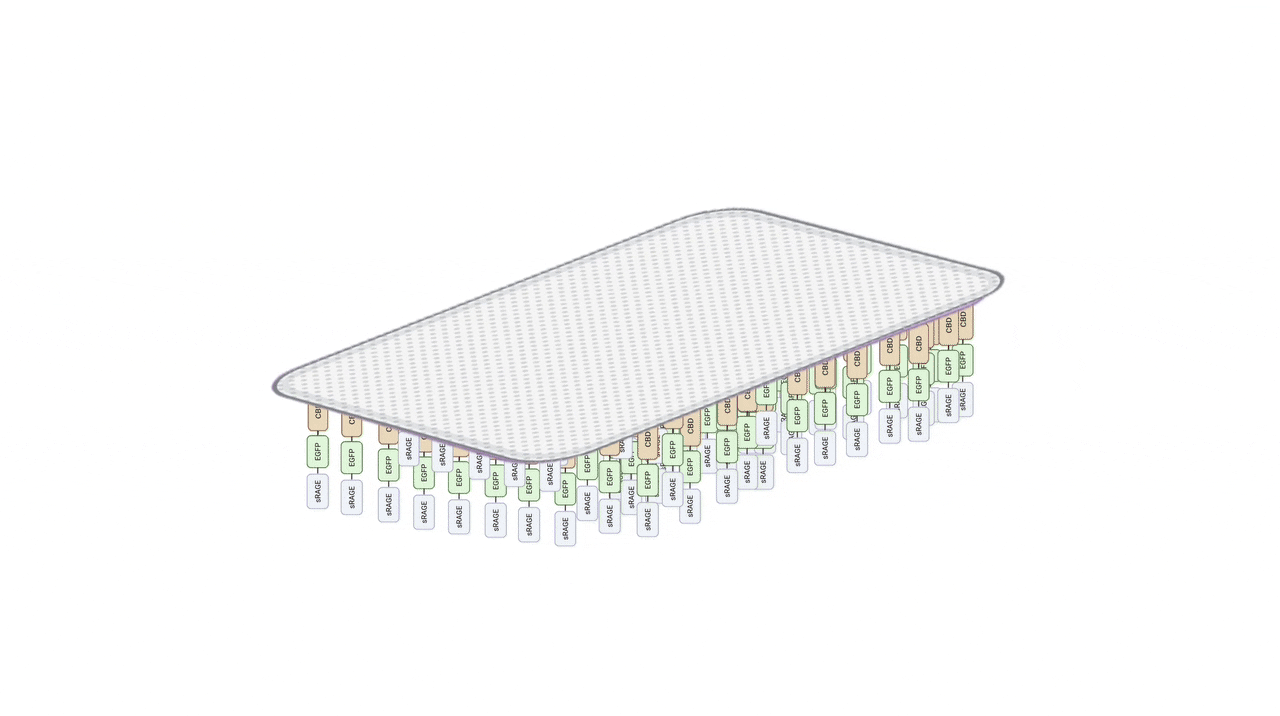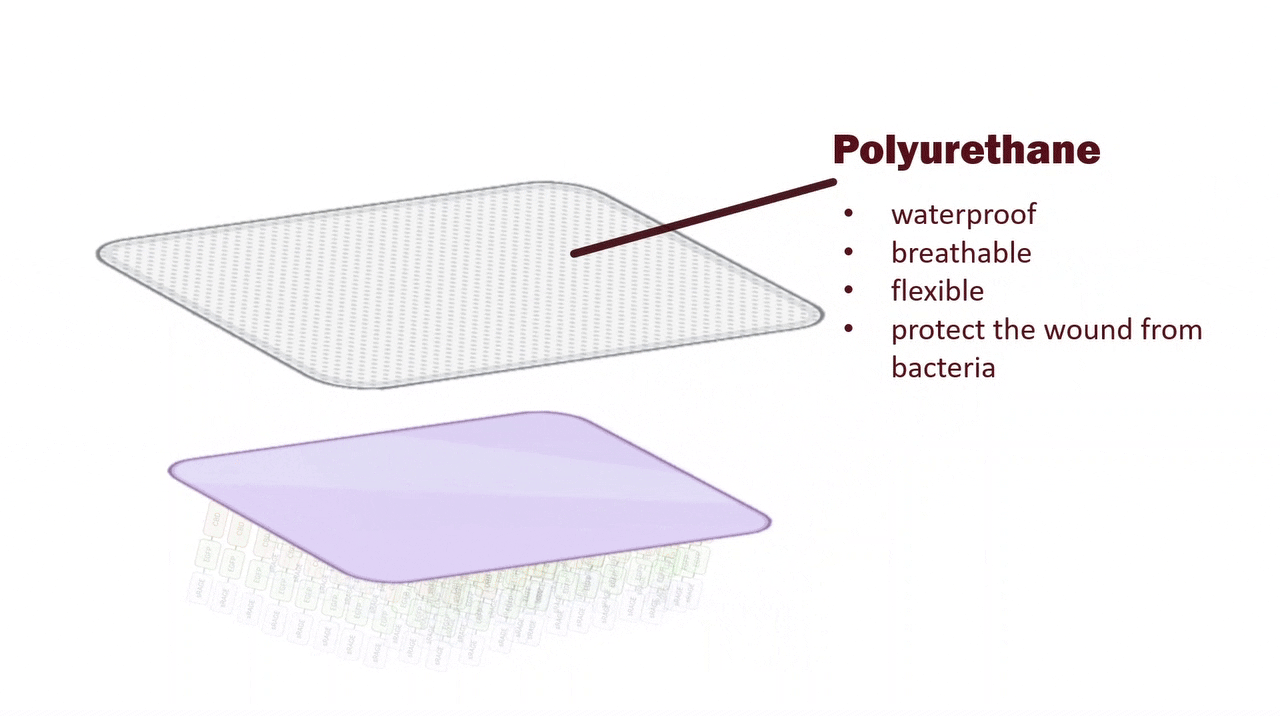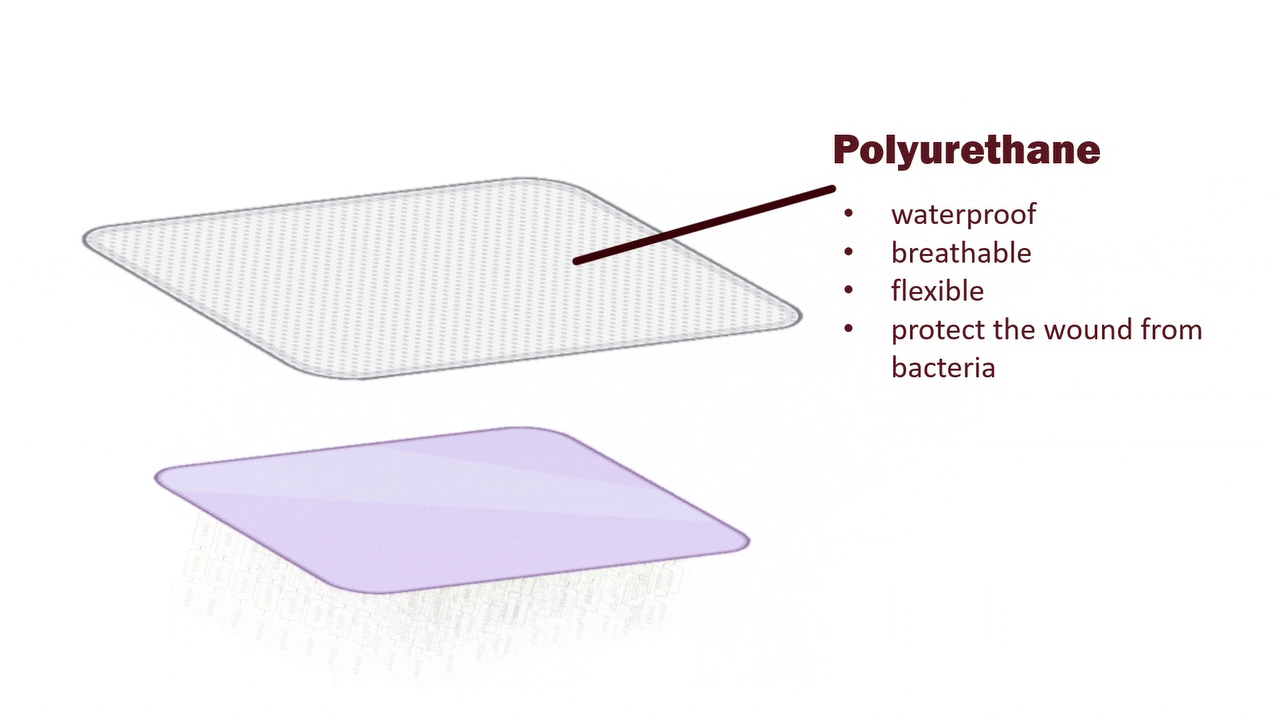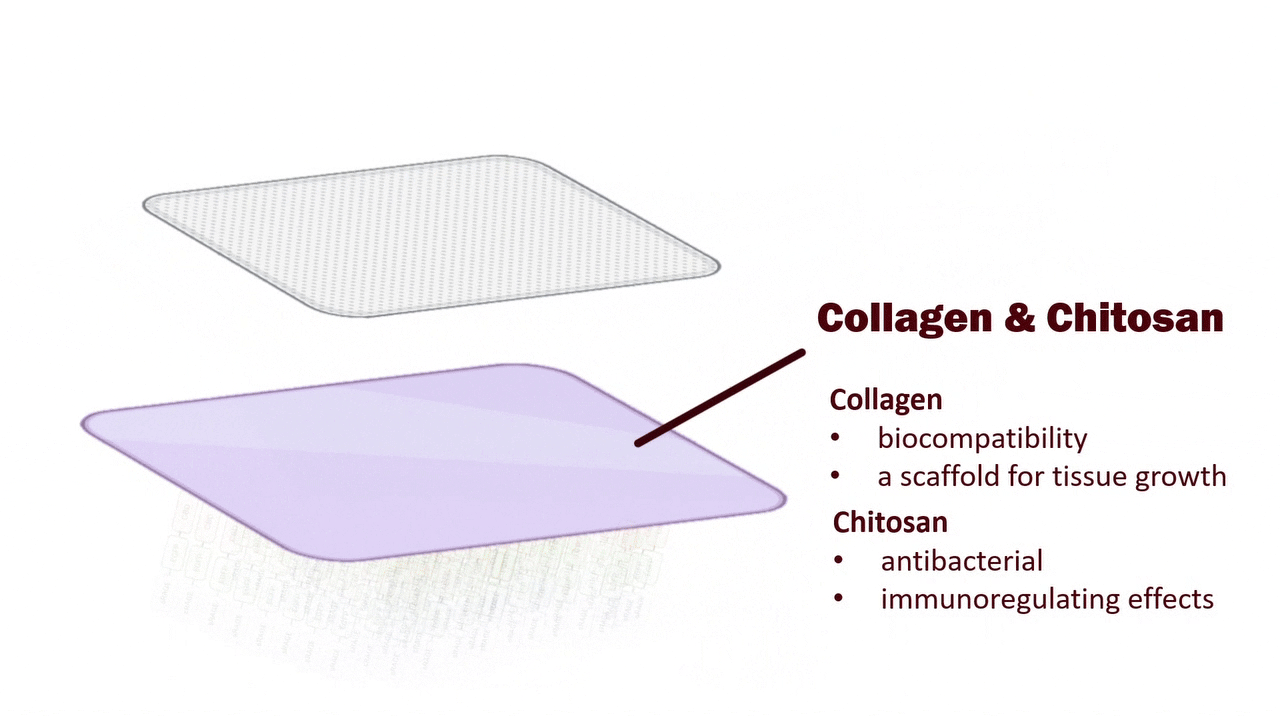
Collagen–Chitosan Hydrogel: A Multifunctional Wound Dressing for sRAGE Immobilization, Antibacterial Protection, and Promotion of Tissue Regeneration
The contact layer is a hydrogel made from a mixture of collagen and chitosan.
Collagen, in direct contact with the wound, mimics the extracellular matrix, providing essential biological signals for cell adhesion, migration, and proliferation. It also promotes angiogenesis, collagen deposition, and accelerates tissue regeneration and re-epithelialization. Importantly, collagen serves as a scaffold to immobilize sRAGE through CBD binding. (Fleck, C. A. 2010)
Chitosan, on the other hand, possesses natural antibacterial activity: its positive charges interact with bacterial cell membranes to inhibit the growth of a broad spectrum of bacteria and suppress biofilm formation. Moreover, its strong exudate-absorbing and gel-forming abilities help maintain a moist wound environment while removing excess fluid, preventing the wound from being soaked. (Bano, I., et al, 2017)
By combining collagen and chitosan into a single hydrogel, we do not simply preserve their individual effects but also enhance moisture retention and wound healing capacity. (Zhang, et al, 2021) By adjusting the collagen-to-chitosan ratio, which modulates the hydrogel’s microstructure, we can optimize its capacity to immobilize sRAGE while retaining antibacterial functionality—thus defining the most effective composition for our collagen–chitosan hydrogel patch (Devernois, J., et al, 2023).

Conclusion
In our AGEs-Thwart design, we integrated both protein engineering and material science to create a modular and clinically adaptable therapeutic system for diabetic foot ulcers (DFU). On the protein level, we engineered sRAGE with enhanced AGE-binding affinity, coupled it with a collagen-binding domain (CBD) for stable immobilization, and further introduced the Npu DnaE split intein system to enable modular ligation of therapeutic proteins. On the material level, we developed a bilayer patch composed of medical-grade PU film and a collagen–chitosan hydrogel, providing antibacterial protection, moisture retention, and biological support for tissue regeneration.
Together, these two systems achieve our primary goal: a sustained and adaptable AGEs-capturing wound patch that can both mitigate AGE-induced inflammation and promote tissue repair. Beyond the project itself, our modular intein-based framework provides a universal platform for protein immobilization, which can be reused and adapted by future iGEM teams for diverse biomedical applications such as antimicrobial, angiogenic, or growth factor delivery.
In the future, we plan to perform in experimental of specific AGE species interacting with our fusion proteins, optimize the hydrogel’s properties, and expand the system’s capability to integrate multiple therapeutic modules for wound care.
Reference
- Zhou, Mengzhou, et al. (2024) Activation and modulation of the AGEs-RAGE axis: implications for inflammatory pathologies and therapeutic interventions–a review Pharmacological research, 206, 107282.
- Osawa, Mari, et al. (2007) De-N-glycosylation or G82S mutation of RAGE sensitizes its interaction with advanced glycation endproducts. Biochimica et Biophysica Acta (BBA)-General Subjects, 1770(10), 1468-1474.
- Kalamajski, S., & Oldberg, Å. (2009) Homologous sequence in lumican and fibromodulin leucine-rich repeat 5-7 competes for collagen binding.Journal of Biological Chemistry, 284(1), 534-539.
- Iwai, H., Züger, S., Jin, J., & Tam, P. H. (2006) Highly efficient protein trans-splicing by a naturally split DnaE intein from Nostoc punctiforme.FEBS letters, 580(7), 1853-1858.
- Cheriyan, M., Pedamallu, C. S., Tori, K., & Perler, F. (2013) Faster protein splicing with the Nostoc punctiforme DnaE intein using non-native extein residues. Journal of Biological Chemistry, 288(9), 6202-6211.
- Fleck, C. A. (2010). Modern collagen wound dressings: Function and purpose. Journal of Wound Care, 19(4), 175–179.
- Bano, I., et al. (2017). Chitosan: A potential biopolymer for wound management. International Journal of Biological Macromolecules, 105, 1355–1366.
- Zhang, M.-X., Zhao, W.-Y., Fang, Q.-Q., et al. (2021). Effects of chitosan-collagen dressing on wound healing in vitro and in vivo assays.Journal of Applied Biomaterials & Functional Materials, 47(4).
- Devernois, J., & Coradin, T. (2023). Synthesis, characterization and biological properties of type I collagen–chitosan mixed hydrogels: A review. Gels, 9(7), 518.