Implementation plan:
Production:
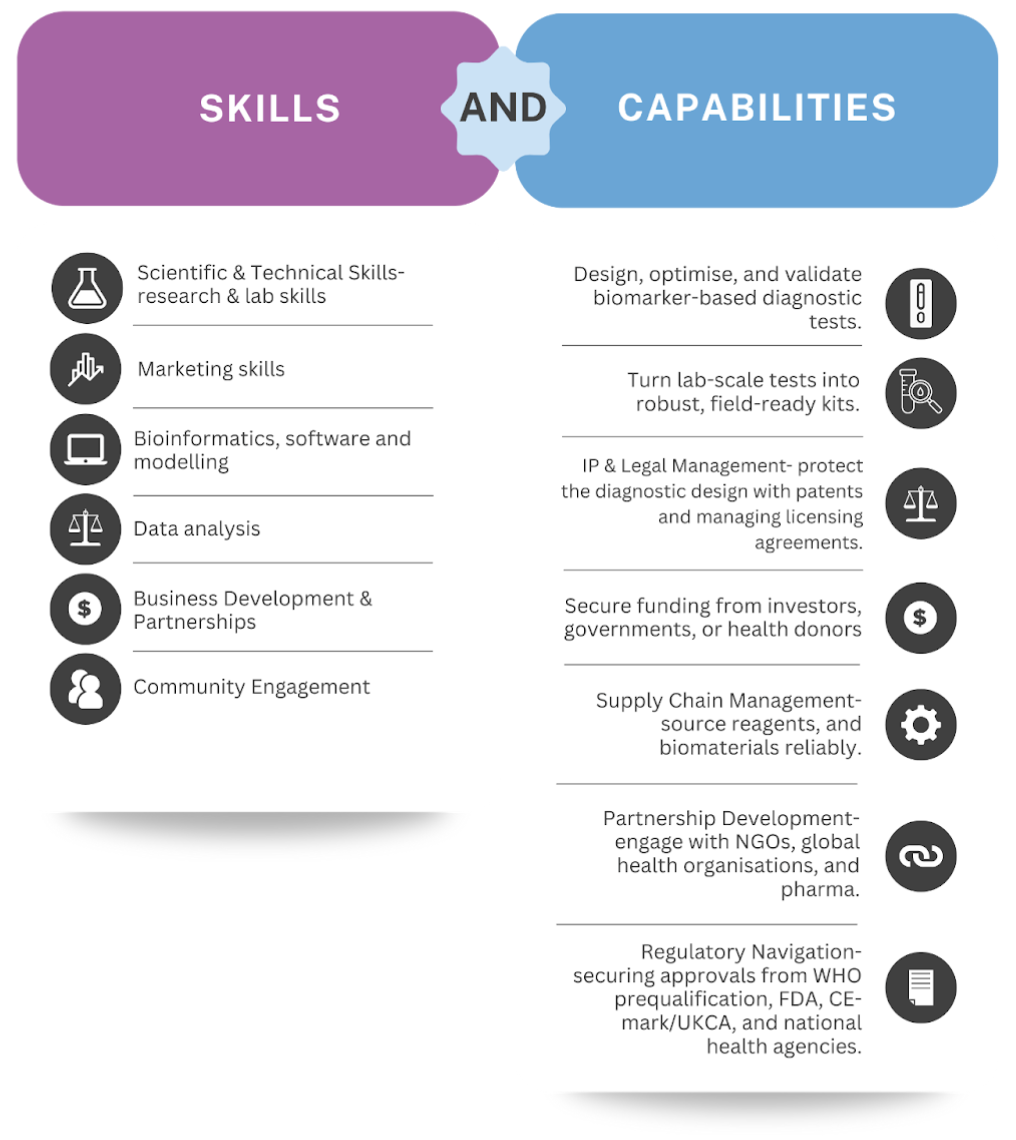
Our production strategy is based on licensing our technology to established diagnostic manufacturers, enabling us to leverage their infrastructure, expertise, and global distribution networks. Prior to production, we will focus on research and development, with continuous testing and refinement throughout multiple engineering cycles. A primary goal will be to enhance test specificity as new biomarker research becomes available. Once our product fully meets all criteria, we will engage in production partnerships. This approach reduces the need for substantial capital investment in internal facilities, thereby lowering financial risk and enabling rapid scaling.
Licensing agreements will ensure our intellectual property is protected while allowing manufacturers to produce the test at scale in compliance with stringent quality standards. Furthermore, licensing generates a sustainable revenue stream through royalties, providing financial stability and supporting ongoing innovation and pipeline expansion. This strategy also offers flexibility, allowing us to partner with manufacturers in various regions and adapt production and distribution to local requirements. Ultimately, licensing enables us to focus on innovation, clinical validation, and expanding our pipeline, while trusted partners manage large-scale manufacturing and logistics, accelerating the widespread availability of our test.
Skills and capabilities required for product production:
Validation:
Before distributing the test product, we need to implement a validation protocol. This will involve sampling products from the manufacturing line to ensure they meet all necessary requirements and function as intended. Additionally, during the initial stages of product development and validation, we will conduct clinical trials to confirm that our test functions effectively not only in the laboratory but also in real-world scenarios.
In order to test the efficacy of any detection system these three criteria should be met:
- The system does not produce a signal when there is no biomarker
- The system does not produce a signal when there is a similar but wrong biomarker
- The system produces a detectable signal when the correct biomarker is present
To test these three criteria, the following steps will be carried out:
- Test the system's expression in the absence of miRNA (also referred to as leakage testing). This will help determine if the system is suitable for further experiments. Some leakage is expected, but it should be minimal (barely visible to the naked eye) to avoid false positives.
- Once the system is confirmed to exhibit minimal leakage, proceed to test it with a non-target miRNA that is up to 80% similar to the intended biomarker. In this case, expect some level of signal expression, but it should be sufficiently low to prevent a false positive response.
- Finally, the system is tested with the target miRNA to confirm that it can generate a clear signal when the target molecule is present.
In our product, the mRFP reporter gene serves as our signal, producing a deep red color upon transcription. We quantify all tests using visible light spectroscopy.
Distribution:
As a start-up, we plan to outsource our product distribution to a partner who will handle the logistics in our initial target country, the Dominican Republic. After consulting with stakeholders, we have concluded that the most effective partnerships would be with NGOs such as FIND, which specialises in global diagnostic distribution in critical locations, and the Global Fund, which focuses on TB prevention and has extensive experience in distributing infectious disease tests.
In low-income countries (LICs), we envision a distribution model that prioritises accessibility, affordability, and integration with existing healthcare networks. Instead of relying solely on central hospitals or specialised laboratories, our test will be distributed through NGO-led outreach programs to ensure it reaches underserved populations. The test is designed to be portable, low-cost, and easy to use without specialised equipment, allowing deployment in both rural and urban areas with limited infrastructure. Collaborating with governments, global health organisations, and NGOs will be central to this strategy, enabling bulk procurement and subsidised pricing to reduce the financial burden on individuals. This approach increases diagnostic coverage and helps overcome barriers such as distance, cost and stigma by bringing testing closer to the communities most in need.
Storage:
Our diagnostic test kits will be stored at normal room temperature in a clean, dry environment to ensure their stability and reliability. They will remain in their original packaging, which protects them from light, dust, and minor physical damage during storage and handling. This simple storage requirement eliminates the need for costly cold-chain logistics or specialised facilities, making the tests highly practical for use in low-resource and urban settings. Keeping the tests in their protective packaging until use, further ensures ease of distribution and preservation of product quality. Our test has a shelf life of three months.
Testing:
The patient will start the test by providing a small blood sample from a simple finger prick, which is then placed into a microfluidic cartridge. The microfluidic system guides the sample through tiny channels that filter, prepare, and concentrate the target biomarkers, ensuring only the relevant components are left. After processing, the sample is transferred onto a paper-based lateral flow strip, where it reacts with reagents designed to detect latent TB RNA biomarkers. If these biomarkers are present, a clear, easy-to-read colour change appears on the strip, giving the patient a simple positive or negative result without needing specialised equipment or training. This combination of microfluidics and lateral flow technology ensures precision and simplicity, making the test highly suitable for low-resource settings. The entire diagnostic process will take 15 minutes to complete.
Upscaling:
Upscaling for our business will focus on transitioning from small-scale prototype production to mass manufacturing while maintaining affordability, quality, and accessibility. This will involve moving from lab-based assembly to standardised, automated production processes that comply with international quality standards such as ISO 13485 and Good Manufacturing Practices (GMP). By partnering with experienced manufacturers through licensing agreements, we can scale quickly without the heavy upfront investment of building our own facilities. To ensure supply chain resilience, we intend to secure reliable sources of raw materials, including microalgae-based polylactic acid (PLA) for cassettes and key reagents, and establish regional manufacturing hubs to reduce costs and improve distribution efficiency. Simultaneously, we will invest in continuous process optimisation to lower unit costs as volumes increase, keeping our tests competitively priced. This scalable model supports rapid growth in the TB diagnostics market and establishes a foundation for adapting our platform to other diseases, increasing both our reach and long-term commercial potential.
However, we anticipate several challenges in upscaling. Supply chain vulnerabilities, such as reagent shortages or fluctuations in microalgae-based PLA availability, will be managed through diversified suppliers and stockpiling strategies. Regulatory hurdles across different jurisdictions may extend timelines, so we will engage early with regulators and seek certifications like WHO prequalification to facilitate global approvals. Maintaining consistent quality at scale is a potential risk, which we will address through strict quality management systems and regular audits of our manufacturing partners. By proactively addressing these barriers, we can ensure upscaling enhances both our commercial success and global health impact.
Our diagnostic test integrates microfluidics with a lateral flow assay, delivering a scalable platform that merges laboratory-level precision with field-ready simplicity. There is already ongoing research into using Cyclic Chain Displacement Reaction (CCDR) to detect miRNAs for lung cancer using a triple-line lateral flow strip-based platform [1], so by swapping detection targets, the system can be quickly adapted to detect a range of infectious diseases, highlighting its adaptability. This versatility supports a robust product pipeline and enables entry into multiple markets beyond TB diagnostics, positioning our technology as a leading solution for global health diagnostics with strong potential for expansion, licensing, and sustained commercial growth.
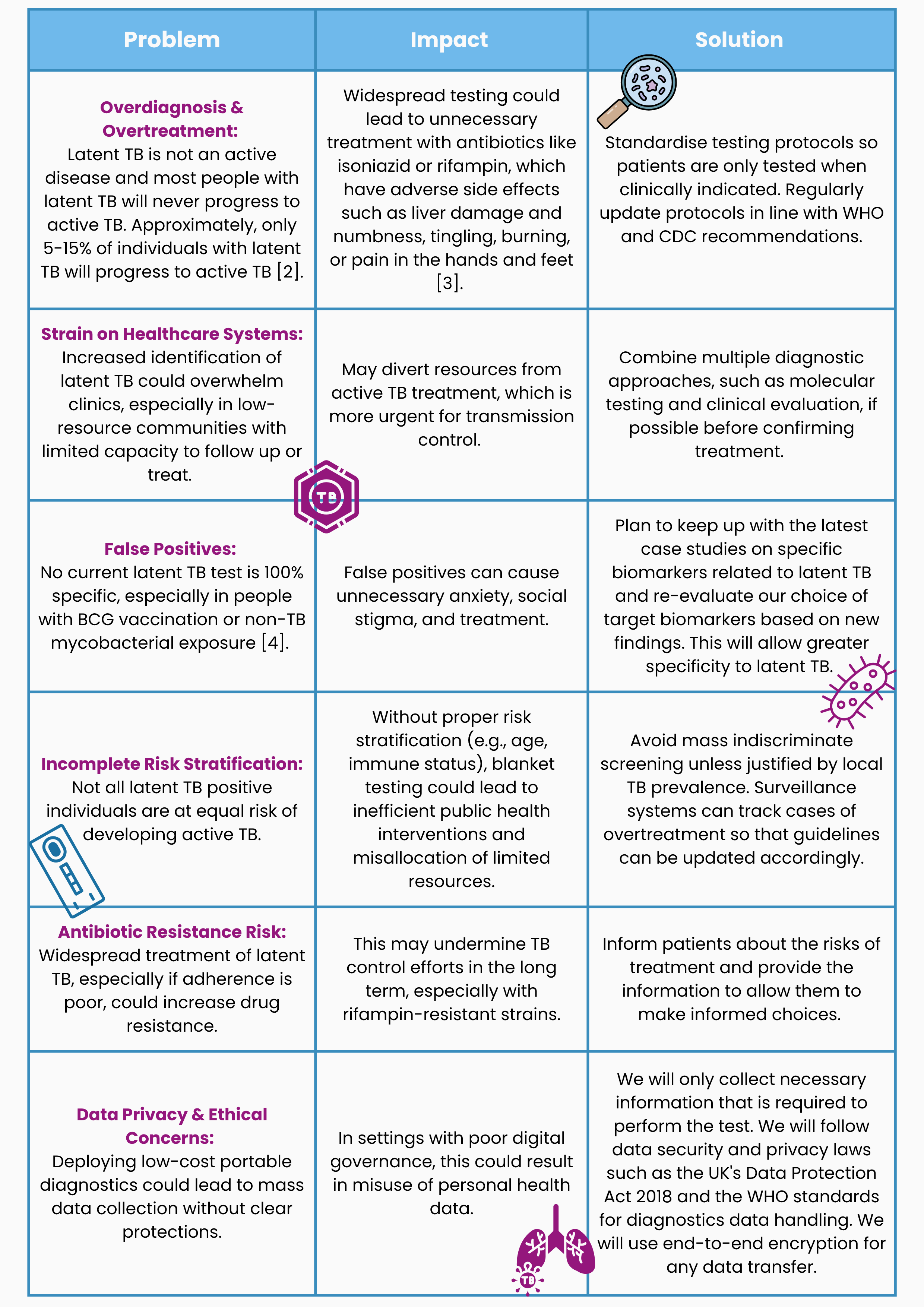
Risk assessment and mitigations:
Earlier detection of latent TB before it becomes active and transmissible, reduces disease spread and lowers TB incidence rates. This can improve treatment outcomes as patients can seek out healthcare options sooner as they will have the knowledge of their health status. Diagnosing patients at the latent TB stage can lower healthcare costs by preventing progression to active TB, which is more expensive to treat as it requires multiple antibiotics over the course of 4-7 months [5]. It will also increase workforce productivity as individuals are less likely to progress to active TB and develop symptoms that directly affect their livelihood.
The simple colour change to provide a yes or no answer helps address misdiagnosis common with existing tests, as it does not rely on a specialist's subjective diagnosis which occurs with a Tuberculin Skin Test. The concept of our test can also reduce stigma, as testing can be simpler, faster, and more discreet. The simplicity and portability of our test reduces dependence on complex lab infrastructure, which will help with the integration into marginalised communities and field settings.
An affordable test reduces financial burden on patients and healthcare systems in communities with low resources and low-income. This makes healthcare more accessible by reducing the financial impact it has on individuals, increasing the likelihood that they will seek testing. Affordability will promote health equity, narrowing the diagnostic gap between rich and poor countries.
Our test uses biodegradable microalgae based PLA which creates a smaller waste footprint [6] compared to non-PLA multi-use plastic consumables in labs. Our portable test does not require reliance on energy-intensive laboratory equipment or electricity. Both of these factors have been put in place to mitigate the environmental effects our product has.
Cross-Sector Impacts:
Pharmaceutical companies:
Our diagnostic test will drive demand for preventive TB treatments, since more latent cases will be detected early. This will increase the income for the pharmaceutical companies however, it will also put a stress on the companies to produce more drugs at a higher rate.
Diagnostic companies:
Once our test has been revised and developed to make it more reliable at testing for a specific infectious disease, it might reduce the reliance on costly lab-based diagnostics. If the health sector moves toward using simpler and cheaper diagnostic tests that have been proven to be as specific and reliable, or even more so, this shift will affect the income of existing diagnostic companies. There are options of partnership with these existing companies to build portfolios and networks that could help our product expand while also providing something to these businesses.
Manufacturing and distribution:
If we expand production and distribution sites in order to reach more locations, this will provide new opportunities for local production and distribution in low-income countries. Providing job opportunities for those living in the area, and helping the economic growth of communities.
Sustainability:
As a start-up, Infecheck considers sustainability a major component of the business model. The actions we will take to achieve these goals are highlighted here in our sustainability plan.
We aim to achieve the goals:



Target 3.3- Our product directly aims to combat tuberculosis, one of the epidemics targeted to be eliminated by 2030, by being a diagnostic test for the detection of latent TB. Detecting latent TB enables individuals to learn if they are carrying the bacteria Mycobacterium tuberculosis before it becomes active and symptomatic. This allows those individuals to seek out early treatment while the disease is still non-transmissible, helping to reduce its spread.
Target 3.8- Our test achieves this by being more affordable, accessible and less complex than current diagnostics used for the detection of latent TB. This supports universal health coverage by allowing those in areas of the world that are most vulnerable to latent TB, which are lower-middle-income countries, to have better access to diagnostic tests. Unlike current tests such as the tuberculin skin test which is considered subjective, our test has an easy to read colour change so there is a definitive yes or no result, which reduces the risk of misdiagnosis.
Target 10.2- In addition to Target 3.8, our more portable and user-friendly test will help individuals in low-income countries access healthcare that may otherwise be out of reach due to financial barriers, or limited resources. Our test also aims to allow access to diagnostics by those who face discrimination by their healthcare system due their ethnicity and sexual orientation. In countries such as the Dominican Republic (our main case study), Haitians are discriminated against healthcare from hospitals so with our plan to make this a test that comes to those who need it this will allow those individuals the same opportunity of diagnosis. Also, in many high-burden countries, TB is stigmatised due to its perceived association with HIV. In some communities, having TB is wrongly linked to being homosexual, which discourages individuals from getting tested out of fear of discrimination or being labelled. Further information on the inequalities surrounding TB care can be found on our Human Practices page.
Target 10.3- Our research into the Dominican Republic and speaking with people from the region (you can find the testimonies here) has had direct influence into how we aimed to create and implement our test. We created a diagnostic test with the goal to reach those who face discrimination by practices conducted in their communities.
Target 12.5- Our paper-based test will be enclosed in a cassette made from microalgae based poly lactic acid (PLA). PLA is widely used in 3D printing for its properties and being a more sustainable alternative as it is biodegradable and made from natural renewable resources. However, its breakdown is very slow and only occurs under industrial, anaerobic conditions [7]; we will ensure we have proper disposal of our product at industrial composting facilities, so that the PLA can be fully biodegraded to its natural components carbon dioxide, water, and biomass, creating nutrient-rich compost which can be used in agriculture. As a start-up, we plan on using microalgae based PLA rather than cane-sugar based PLA. This is because cane-sugar based PLA requires higher land area (959 m2 × a crop eq/tonne PLA) and water demand (183 m3 eq/tonne PLA), whereas microalgae based PLA is non-land-based and can utilise wastewater and CO2 from industrial emissions [8]. This will create a circular life cycle. When 3D printing the cassette with PLA, volatile organic compounds (VOCs) will be released; they are considered non-toxic but can cause respiratory irritation when exposed to for a prolonged period of time at high levels [9]. The fumes produced when heating PLA during the process of 3D printing are less severe than using Acrylonitrile-Butadiene-Styrene (ABS) [9] as the filament material. We plan to use PLA so that we can reduce the toxic fumes produced for safer exposure when printing the cassette.
References:
- Zhou P, Lu F, Pan W, Yin J, Li N, Tang B. Cyclic chain displacement amplification-based dual-miRNA detection: a triple-line lateral flow strip for the diagnosis of lung cancer. Chemical Communications [Internet]. 2021 Jan 1;57(92):12301–4. Available from: https://pubs.rsc.org/en/content/articlelanding/2021/cc/d1cc05442b
- Price C, Nguyen AD. Latent tuberculosis [Internet]. StatPearls - NCBI Bookshelf. 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK599527/
- Rifampin and isoniazid (oral route) [Internet]. Mayo Clinic. 2025. Available from: https://www.mayoclinic.org/drugs-supplements/rifampin-and-isoniazid-oral-route/description/drg-20062747
- Nayak S, Acharjya B. Mantoux test and its interpretation. Indian Dermatology Online Journal [Internet]. 2012 Jan 1;3(1):2. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC3481914/
- Belknap R, Haas M. Updates in the treatment of active and latent tuberculosis. Seminars in Respiratory and Critical Care Medicine [Internet]. 2018 Jun 1;39(03):297–309. Available from: https://pubmed.ncbi.nlm.nih.gov/30071545/
- Chen G, Li J, Sun Y, Wang Z, Leeke GA, Moretti C, et al. Replacing Traditional Plastics with Biodegradable Plastics: Impact on Carbon Emissions. Engineering [Internet]. 2023 Nov 22;32:152–62. Available from: https://doi.org/10.1016/j.eng.2023.10.002
- Toor R. How Sustainable is PLA 3D Printer Filament? [Internet]. Filamentive. Available from: https://www.filamentive.com/how-sustainable-is-pla/
- Environmental footprint of polylactic acid production [Internet]. Research portal. Available from: https://researchportal.scu.edu.au/esploro/outputs/journalArticle/Environmental-footprint-of-polylactic-acid-production/991013264076802368#file-0
- Doan MC. Is PLA filament fume toxic to markers? [Internet]. Alveo3D. 2024. Available from: https://www.alveo3d.com/en/pla-filament-fume-toxic-3d-printing/
