Overview
We conducted two parallel sets of DBTL cycles:
- We conducted research on bacteria engineered to produce curli fusion proteins, revealing potential oligomerization issues in curli fusion proteins.
- We successfully created biomineralized samples utilizing S. pasteurii’s natural biomineralization capabilities
Initial Considerations
Our goal was to design bacteria that would simultaneously produce a sticky biofilm and induce biomineralization, capturing microplastics and forming an aggregate usable in construction. We chose to use curli fibers, a key component of E. coli biofilms, because they exhibit high adhesive properties and strong tensile mechanical properties (Zhang et al., 2017). Examining pathways involved in Microbially Induced Calcite Precipitation (MICP), we found that bacterial strains utilizing the urea hydrolysis pathway showed more calcite precipitation (~20–80%) in comparison with other metabolic pathways (Castro-Alonso et al., 2019). This pathway is primarily driven by the enzymes urease and carbonic anhydrase. To simultaneously produce curli fibers and induce biomineralization, we decided to emulate the fusion protein of curli fiber and amylase that Neel Joshi’s Biologically Fabricated Materials Lab designed (Birnbaum et al., 2021). Since carbonic anhydrase is a monomeric enzyme in the urea hydrolysis pathway, it was natural to design and experiment with a curli fiber and carbonic anhydrase fusion protein. We chose to use the strain E. coli K12 to express our fusion protein, as E. coli K12 can, under the right conditions, produce both curli fiber and carbonic anhydrase.
Our second goal was to create concrete biomineralized products. We chose to use S. pasteurii, which naturally has a urea hydrolysis pathway that induces calcium carbonate precipitation. We used sand to act as a substrate and scaffold for mineralization.
DBTL Cycles: Curli + MICP
Overview of part: Curli + sazCA
The goal of this part was to simultaneously produce curli fiber and induce biomineralization using one fusion protein. The 2023 iGEM team MSP-Maastricht’s biomineralization carbonic anhydrase module inspired us to use sazCA, which is also the fastest carbonic anhydrase to date (Y. Zhu et al., 2021). As such, our final part’s primary goal was to produce a csgA-sazCA fusion protein.
DBTL Cycle 1
Design:



To simultaneously produce curli fiber and induce biomineralization, we designed a part BBa_25A8Q07G that could produce and export a fusion protein of csgA, the primary curli monomer, and sazCA, the fastest carbonic anhydrase to date. Our designed curli fusion protein emulates the curli fusion proteins from Neel Joshi’s Lab (Birnhaum et al., 2021), connecting csgA to sazCA via a flexible linker. We inserted our composite part BBa_25A8Q07G into the Twist vector pET blank (Amp), creating the plasmid BBa_25J7AMCD. Thus, our transformants would express ampicillin resistance and would express our curli-sazCA fusion protein in the presence of IPTG.
Build:
Once our plasmid was synthesized by Twist, we performed chemical transformation on two tubes of wild E. coli K12, one of which we added our plasmid pET-csg-sazCA-amp. We recovered and plated the transformed cultures on both LB plates and LB/amp plates, letting the plates incubate overnight at 37C.

Top right: LB/amp plate, K12 with added DNA
Bottom left: LB plate, K12 without added DNA
Bottom right: LB plate, K12 with added DNA
As hypothesized, for our bacteria without plasmid DNA, there was a smear on the LB plate and no bacteria on the LB/amp plate. For our bacteria with plasmid DNA, there was a smear on the LB plate and individual colonies on the LB/amp plate. This confirmed that our ampicillin was working properly, and that our E. coli K12 had likely been transformed.
We further confirmed the transformation of our E. coli by performing colony PCR and sequencing via Plasmidsaurus.

The presence of dim bands around 4kb indicates the presence of our plasmid in all three transformant cultures.
Test:
Our first step was to test a range of IPTG concentrations to find the optimal concentration for inducing protein expression. To test protein expression, we used a congo red fluorescence assay inspired by Kan et al. (2019) as well as an absorbance-based spin-down assay from the iGEM Delft-Leiden team.


From these results, we decided to proceed with an inducer concentration of 0.2 mM.
With our transformants, we then performed a congo red fluorescence assay to test for the presence of curli fibers. Congo red binds to amyloid fibrils like those found in curli fibers, producing more fluorescence at 525nm excitation and 625nm emission when bound to these fibrils. We induced each of our cultures with 0.2 mM iPTG and ran a congo red fluorescence assay.

This assay supported our positive control expressing curli, but indicated to us that our transformants were struggling to express curli. Given that our transformants were confirmed by sequencing, we suspected that our protein had issues folding.
Learn:
Using Alphafold 3, we modeled the oligomerization of our modified curli fiber as compared to our positive control, and saw significant differences in folding.

DBTL Cycle 2
Design:
Having a suggestion from the Dry Lab, we designed a Wet Lab based experiment to confirm if issues were happening at the protein folding stage or later.
To do this we designed individual IPTG inducible constructs with our proteins, curli and carbonic anhydrase. Our goal was to confirm that expression of each individual protein was successful, which would support the conclusion that the problem was unique to the modified protein.


We additionally planned a reverse transcription PCR (RT-PCR) assay. RT-PCR would allow us to view the amount of mRNA produced from a given construct, via first converting it to cDNA and then amplifying it. This would potentially confirm that transcription was present in our modified construct, and support that the issue lied at the translation/protein folding stage.
In designing RT-PCR assay, we included a positive control of the RSS housekeeping gene in E.coli to confirm the validity of our assay.
Build:
We utilized our previously made competent cells to create transformants of both constructs. We ordered reagents required to run a RT-PCR assay, including custom primers and Reverse Transcriptase.
Test:
We ran a congo red fluorescence assay on both our Curli only construct, confirming curli individually did express successfully in E.coli K-12 and that the problem resided in the fusion protein.

The RT PCR gel showed mRNA expression was present, indicating the issue had to do with protein folding further down the line. The bright lines below 1 kb show transcription of the housekeeping gene in all samples. The lines between 3 and 4 kb show curli expression.

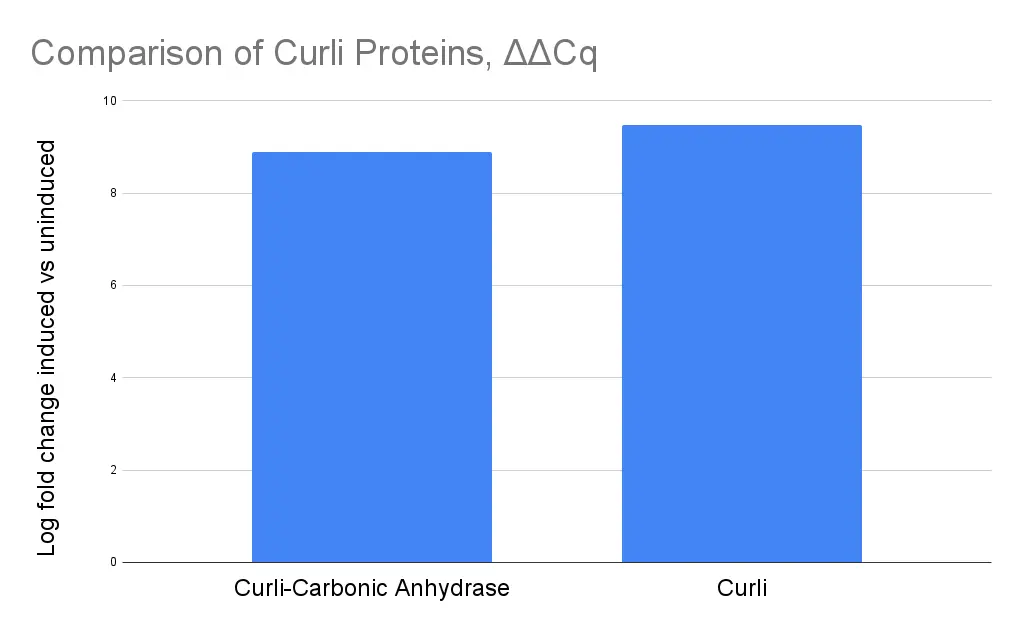
To further quantify RNA expression of our genes relative to the rssA housekeeping gene, we ran RT-qPCR utilizing Bioneer’s accupower greenstar qPCR mix. ΔΔCq values were plotted, and the results showed highly similar induction across csg-sazCA gene cluster (BBa_25A8Q07G) and the unmodified curli operon (BBa_25A80I7T). This further supports that lack of expression resulted from issues that were post transcriptional.
Finally, we also tested expression of carbonic anhydrase through the Wilbur Anderson assay 2023 iGEM team MSP-Maastricht. Unfortunately, we ran into issues with continuously reducing measurements from when our CO2 solution was opened, a known issue with the assay. In the limited time we had remaining, we were unable to further optimize the assay to see usable results.
Learn:
Through both expression of unmodified proteins and RT-PCR data, we were able to support our hypothesis that the issue with our modified construct occurs at the protein folding/oligomerization stage.
Overview of Part: Curli + Urease
As soon as we discovered that there may be potential folding issues with our curli fiber and carbonic anhydrase fusion protein, we switched tracks to design a part with urease instead of carbonic anhydrase. Unfortunately, due to time constraints, we were unable to make it past the design and build stage.
DBTL Cycle 3: Curli + Urease
Design:
As soon as we discovered that there may be potential folding issues with our curli fiber and carbonic anhydrase fusion protein, we switched tracks to design a part with urease instead of carbonic anhydrase. Unfortunately, due to time constraints, we were unable to make it past the design and build stage.

This construct attached the urease gene cluster to the sequence after the curli fiber gene cluster. The urease gene cluster was taken from part BBa_K5205014, designed by the 2024 Hangzhou-SDG iGEM team. We planned to create this construct by inserting the urease gene cluster into one of our existing curli plasmids.
As we received a grant from Ansa Biotechnology to synthesize a plasmid with less than 10kb of insert, we ended up designing a subsequent construct that used arabinose instead of IPTG to induce expression.

Build:
We ordered the urease gene cluster in two gene fragments synthesized by IDT, with the plan to ligate them via NEB HiFi DNA Assembly and insert into our plasmid pET_csg_Amp via restriction enzyme digest. At the end of September, we also received our construct from Ansa Biotechnology. Once we had transformed bacteria, we planned to assess curli fiber presence via congo red fluorescence assays and urease activity with a phenol red pH assay (protocol).
Unfortunately, due to time constraints and our focus on characterizing csg-sazCA, we were unable to transform and perform experiments on this particular construct.
DBTL Cycles - S. pasteurii
Overview: S. pasteurii
In parallel with our main experimental DBTL cycles, we conducted experiments to create a rock-like material and provide a proof of concept. We chose to work with S. pasteurii, a naturally biomineralizing bacteria, as it naturally utilized the urea hydrolysis pathway. We procured S. pasteurii and the necessary protocol from Kelsey De Frates, from the Hernandez Lab at UCSF.
DTBL Cycle 1:
Design:
To create a biomineralized hard product that could be tested, we designed a mold following ASTM standards for tensile/compressive strength testing with scaled down dimensions. The resulting product had a cross-sectional area of 2 squared, allowing us to later estimate tensile strength.

Build:
We then built a PDMS mold from a 3D printed PETG inverse based on our CAD model, following our protocol “Manufacturing PDMS Channel-Pore Microfluidic Devices.”

In parallel, we cultured S. pasteurii from glycerol stocks at 30C. Having acquired the appropriate mold, media, and S. pasteurii culture, we began biomineralization, following protocol from Troyer et al. Our three molds, one which was our PDMS mold and the other two which were normal 3D molds, were filled with sand to act as nucleation sites. For one month, we conducted daily interchange between S. pasteurii culture and precipitation media to induce calcite precipitation.
Test:
Samples were then taken out of molds and sterilized as per our sterilization protocol. We were able to confirm that our protocol successfully sterilized our samples via a swab test. While the process of removing the samples from our molds broke the mineralized material into multiple pieces rendering us unable to measure strength, we can see that the sand has clearly turned into a much more coarse material.

Learn:
From this process, we learned that fine-grain sand worked best for biomineralization. While creating a biomineralized product, we encountered many contamination issues. Since our S. pasteurii cultures had slower growth rates, any contamination of other bacteria or yeast outcompeted our S. pasteurii cultures. It took us a couple of tries to successfully biomineralize, through which we learned a variety of techniques to ensure maximal sterility, including daily transfer cultures, occasional new S. pasteurii cultures from glycerol stocks, and a healthy dose of paranoia.
References
Birnbaum, D. P., Manjula‐Basavanna, A., Kan, A., Tardy, B. L., & Joshi, N. S. (2021). Hybrid living capsules autonomously produced by engineered bacteria. Advanced Science. 8(11). https://doi.org/10.1002/advs.202004699
Castro-Alonso, M. J., Montañez-Hernandez, L. E., Sanchez-Muñoz, M. A., Franco, M. R. M., Narayanasamy, R., & Balagurusamy, N. (2019). Microbially Induced calcium carbonate precipitation (MICP) and its potential in bioconcrete: Microbiological and Molecular concepts. Frontiers in Materials, 6. https://doi.org/10.3389/fmats.2019.00126
Kan, A., Birnbaum, D. P., Praveschotinunt, P., & Joshi, N. S. (2019). Congo red fluorescence for rapid in situ characterization of synthetic curli systems. Applied and Environmental Microbiology, 85(13), e00434-19. https://doi.org/10.1128/AEM.00434-19
Zhang, Y., Wang, A., DeBenedictis, E. P., & Keten, S. (2017). Bending energy penalty enhances the adhesive strength of functional amyloid curli to surfaces. Nanotechnology, 28(46), 464002. https://doi.org/10.1088/1361-6528/aa8f72
Zhu, Y., Liu, Y., Ai, M., & Jia, X. (2021). Surface display of carbonic anhydrase on Escherichia coli for CO2 capture and mineralization. Synthetic and Systems Biotechnology, 7(1), 460–473. https://doi.org/10.1016/j.synbio.2021.11.008
Troyer, E., Berninghaus, A., Gerlach, R., Foreman, C., Joyce, J., West, C., and A. J. Phillips. "Biomineralized Art: Using Microbes and Minds to Make Mountains." Paper presented at the 51st U.S. Rock Mechanics/Geomechanics Symposium, San Francisco, California, USA, June 2017.