Introduction
Envenomation by poisonous or venomous animals represents a persistent global health challenge. Although many species across various phyla possess toxic potential, only a limited number cause significant harm to humans or domestic animals. Each year, millions of envenomation incidents are reported worldwide, typically associated with relatively low mortality rates (Acevedo & Escobar, 2025; Mebs, 2006). Most phyla have a few representative genera with the capacity to generate toxins, with few species representing a health concern. Among venomous taxa, snakes and scorpions pose the greatest threat to human health. Scorpion stings account for approximately 1.2 million cases annually, with fatality rates below 1% (0.27% for Mexican species) (Acevedo & Escobar, 2025; Ahmadi et al., 2020; Chippaux & Goyffon, 2008). On the other hand, snakebites cause an estimated 3 million envenomations each year, with 1–5% mortality depending on the species (Acevedo & Escobar, 2025; Casewell et al., 2020). Jellyfish are possibly the largest cause of intoxication by sting with an estimated 150 million cases each year, however they are by far the least lethal with between 20 and 100 deaths in the same time period (Osathanunkul et al., 2022).
There are two types of animal toxins when classified by chemical origin: peptides and small chemical molecules (SCM). These two groups share metabolic and evolutionary origins within themselves. SCM are usually utilized as defense or are a metabolic byproduct. They are not produced by animals but by plants or bacteria. These toxins are hard to denaturalize by heat and have diverse metabolic origins. Certain species of animals developed methods to accumulate SCM toxins in their body, making them poisonous (intoxication is caused by contact or consumption of the animal). Species like fugu and the poison dart frog are examples of animals with SCM toxins (Acevedo & Escobar, 2025; Chen et al., 2018; Hodgson, 2004; V et al., 2021). On the other hand, peptides evolved as a predatory mechanism. Toxic proteins are metabolically taxing (although not as taxing as the complex enzymatic networks necessary to produce toxic secondary metabolites), as such they competed during evolution with other hunting mechanisms like size. Spiders, snakes and scorpions all have protein-based toxins (Acevedo & Escobar, 2025; Chen et al., 2018; Mebs, 2006). While intoxication by SCMs is harder to cure, it is also rarer and easier to prevent. SCMs intoxication usually happens by mishandling or consumption of the animal, like with the Japanese fugu. SCMs are deadlier than peptides, with smaller LD50s (Lethal Dose, 50%. It is a toxicological measure that indicates the amount or concentration of a substance — such as a venom, toxin, or chemical — required to cause death in 50% of a tested population) quicker action time and harder to implement treatments (Acevedo & Escobar, 2025; Chen et al., 2018).
Since most cases of intoxication by animals are caused by species with peptide-based toxins there is interest in the development of efficient, safe, and cost-effective treatments, especially since SCM intoxication can be largely addressed with safety standards and prevention (Alonso Villela et al., 2023; Bermúdez-Méndez et al., 2018). Peptide animal toxins (PAT) are not as likely to cause long term harm to humans since most animals evolved to deliver doses to kill animals smaller than a house cat (Fry, 2005; Ma et al., 2012).
While there are many species of animals with toxic potential, only a small fraction of them presents a threat to humans, Table 1.
Table 1. Medically significant venomous animal species
| Type of animal | Number of recorded species | Number of species dangerous to human health | Source |
|---|---|---|---|
| Snakes | 3971 | 600 | ResearchGate (2024) |
| Scorpions | ≈2,000 | ≈35 | Romero-Moreno et al. (2023); Mabunda et al. (2024) |
| Jellyfish | >2000 | 13 | Osathanunkul et al. (2022) |
| Spiders | 45000 | 12 | Rahmani et al. (2014) |
The protein nature of PATs and their relatively low lethality in large mammals enable therapeutic intervention. Traditional FDA-approved antivenom production methods involve administering small quantities of harvested venom cocktails from relevant local species to large animals (typically horses) (Bermúdez-Méndez et al., 2018; Rodríguez et al., 2015). The animal's immune system generates antibodies that bind and neutralize the toxins, preventing lethal damage. These antibodies persist in the animal's circulation, allowing serum extraction from blood for antivenom formulation (Bermúdez-Méndez et al., 2018).
However, there are several challenges in using animal-derived antivenom. From an epidemiological perspective, species posing the greatest risk to humans predominantly inhabit equatorial regions with warmer climates. Consequently, countries in the Global South with lower development indices face a disproportionate risk (Alonso Villela et al., 2023; Mabunda et al., 2024; Mebs, 2006). Even in developed nations, most envenomation incidents occur in rural and sparsely urbanized areas where healthcare infrastructure is limited (Al-Hanawi & Chirwa, 2021; Shami et al., 2023). These geographical locations face compounding factors, including scarce health facilities (clinics, hospitals, etc.), limited access to specialized medications and physicians—which may need to be transported from nearby cities—and generally lower economic resources. The localized nature of envenomation—lacking the epidemic characteristics of microbial pandemics—may reduce incentives for centralized pharmaceutical companies to invest in antivenom development and implementation of antivenom production/distribution systems (Habib & Brown, 2018; Habib et al., 2020). These challenges are amplified in developing countries where pharmaceutical infrastructure is limited and economic inequalities are pronounced. Other types of medication can be imported in full or in part while local infrastructure is being developed. However, the cost of production, localized demand, and logistical constraints may limit or impede investment (Habib et al., 2020; Habib & Brown, 2018). Additionally, from the perspective of pharmaceutical companies, investing in antivenom development is costly and risky. Antivenoms are subject to variable demand that changes with the seasons, have a limited profit margin, and require large upfront investments. This results in a phenomenon known as the antivenom cycle (Figure 1), in which demand for antivenoms is unmet due to complications in the market (Antivenoms, n.d.).
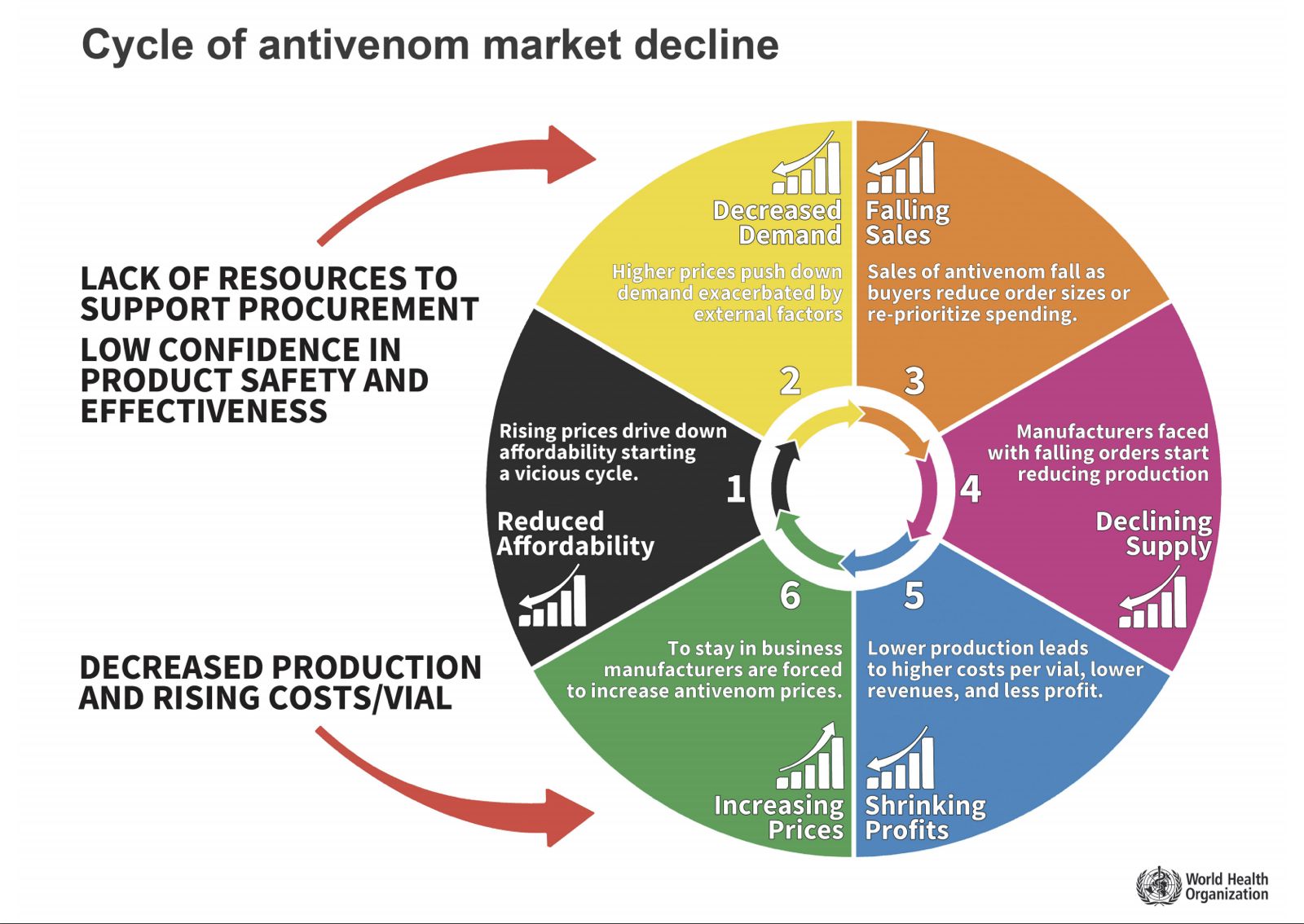
Figure 1. Antivenom market Cycle (World Health Organization, 2025).
Given these challenges and the protein-based nature of PAT therapeutics, microbial-based production systems have been proposed as alternatives. While microbial production of recombinant antivenoms offers multiple potential benefits, no such system is currently in commercial use (Habib & Brown, 2018; Riaño-Umbarila et al., 2025). Existing microbial-based production of therapeutic recombinant proteins typically involves single, relatively small molecules (such as insulin) (Almquist et al., 2014; Kavšček et al., 2015; Rodríguez et al., 2015; Stech et al., 2014). Translating production of antibody collections from animals to cell cultures imposes substantial metabolic demands and generally requires complex mammalian cell cultures, increasing costs. For recombinant antivenoms to compete successfully with traditional alternatives, they must demonstrate competitive effectiveness and cost-efficiency.
Several strategies have been proposed to address these challenges. First, the use of smaller functional molecules containing the critical binding regions of full antibodies, such as single-chain variable fragments (scFvs), offers advantages including reduced metabolic burden on cell cultures, decreased complexity enabling direct expression-secretion systems, and compatibility with bacterial or yeast production (since they don’t require post translational modifications) platforms that are simpler and more economical than mammalian cell cultures (Arauzo-Aguilera et al., 2023; Arias et al., 2017; Ćulah, 2022; Denizci Öncü et al., 2022; Stech et al., 2014). However, since scFv sequences derive from antibodies, they are limited in the number of toxins they can neutralize, and most proposed scFv production systems focus on single antivenoms—making them less competitive than animal-derived antivenoms that can neutralize multiple toxins (Riaño-Umbarila et al., 2025; Riaño-Umbarila et al., 2021).
The second strategy employs machine learning, neural networks, and molecular simulation technologies to generate novel protein sequences with desired characteristics through de novo protein design. These proteins can be designed for polyspecificity (effectiveness against multiple toxins) and optimized for production efficiency (Camacho et al., 2023; Segura et al., 2017). These strategies have different advantages and disadvantages depending on the method used for sequence generation. However, computational models still struggle to integrate all experimental variables, and sequences with strong in silico characteristics may not translate to experimental efficacy (Miller et al., 2022; Hie & Yang, 2022). Additionally, these methodologies can generate hundreds of candidate sequences per run, stretching experimental time and resources (Hie & Yang, 2022; Miller et al., 2022).
The third strategy involves implementing optimized production systems based on highly engineered host organisms and medium-scale bioreactors. This approach recognizes that as research advances in functional therapeutic recombinant protein production, optimized implementation systems become necessary (Behravan et al., 2022; Ćulah, 2022; Riaño-Umbarila et al., 2025). Since therapeutic implementation cycles are lengthy and expensive, demonstrating efficient and cost-effective production of theoretical recombinant antivenoms is essential. Synthetic biology principles—including standardization and modularity—play important roles in designing such systems (Tang et al., 2023; Prielhofer et al., 2017).
Scorpion Envenomation in Mexico
Scorpion envenomation in Mexico follows some global trends while exhibiting unique characteristics. Mexico experiences the world's highest incidence of scorpion stings, with approximately 300,000 cases registered annually (Riaño-Umbarila et al., 2025; Abroug et al., 2020; Riaño-Umbarila et al., 2019). Envenomation rates vary regionally, with half of all cases concentrated in five states: Guanajuato, Jalisco, Guerrero, Michoacán, and Morelos (Riaño-Umbarila et al., 2025; ResearchGate, 2025).
From a taxonomic perspective, all medically relevant Mexican scorpions species (medically relevant meaning that it can pose a risk to human health, since all scorpions are venomous but not all are a threat to human health) belong to the Centruroides genus. While colloquially termed “Mexican scorpions”, this genus only represents 15% of scorpion species found in Mexico, with approximately 20 species considered medically relevant (Jimenez-Flores et al., n.d.).
According to Official Mexican Standard NOM-033-SSA2-2011, scorpion envenomation is classified into three grades based on symptom severity (Hernandez, 2011) (Table 2).
Table 2. Classification of scorpion envenomation severity (Hernandez, 2011)
| Grade 1 Mild | Grade 2 Moderate | Grade 3 Severe |
|---|---|---|
|
Local pain Local paresthesia (local tingling) Pruritus (itching in the affected area) Mild restlessness |
Anxiety Itching in the nose, mouth, and throat Rhinorrhea Sialorrhea Foreign body sensation in the pharynx Dysphagia Tongue fasciculations Dry mouth sensation Tachycardia Dyspnea Abdominal distension Abdominal and muscle pain |
High or low blood pressure Fever or hypothermia Photophobia Nystagmus Dyslalia Seizures Bradycardia Arrhythmias Oliguria Unconsciousness Multiple organ failure Coma Death |
Mexican antivenom production began in the early 19th century using dogs before transitioning to horses. The Mexican industry of antivenoms is concerned with providing products against scorpion, snake, and spider envenomation (Boyer, 2013; Chippaux et al., 2020; Mexico, 2025). Given Mexico has the highest global incidence of scorpion stings (one quarter of all registered cases worldwide), scorpion envenomation represents a significant public health concern. While Centruroides scorpions also live in the southern states of the United States of America, the comparative low incidence has not produced a major investment to produce and improve antivenoms in the US. Historically the US (Boyer, 2013; Chippaux et al., 2020).
Early antivenoms were crude formulations, and physicians initially avoided routine use due to safety concerns (Boyer, 2013). Although purification techniques improved during the mid-19th century, a major breakthrough occurred in the 1990s (Boyer, 2013; Chippaux et al., 2020). Significant public and private investment during this decade focused on improving antivenom safety, efficacy, and production scaling, partly motivated by a scorpion sting affecting the president's son when reliable antivenoms were unavailable (National Geographic, 2025).
Antivenom production became subsidized, and extensive public prevention campaigns were implemented (National Geographic, 2025). Advanced purification techniques substantially improved safety. Three major Mexican producers have consistently manufactured over 200,000 doses since the 1990s, scaling to 300,000 doses in the 2000s to match annual sting incidence (Figure 2) (Chippaux et al., 2020; The Eye Mexico, 2025; National Geographic, 2025). While initially focused on scorpion envenomation, similar technologies were applied to snake and spider antivenoms (Chippaux et al., 2020; The Eye Mexico, 2025; National Geographic, 2025). One antivenom from this era received FDA approval in 2011—the first antivenom approved by the agency and the first medicine fully developed in Latin America (University of Arizona News, 2025).
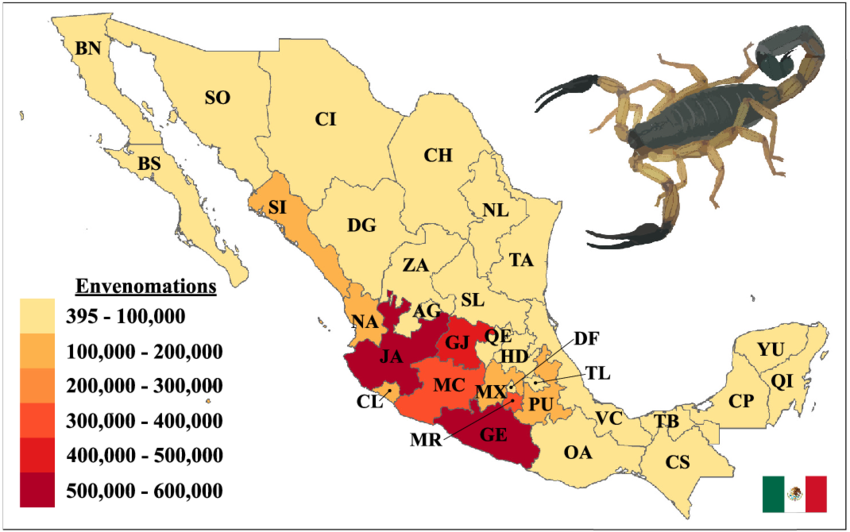
Figure 2.- Scorpion stings in Mexico over the past 10 years (ResearchGate, 2025).
Following these developments and systemic Mexican healthcare improvements, mortality declined dramatically. Scorpion envenomation-related deaths decreased by over 80% between the 1980s and 2000s (Chippaux et al., 2020). Effective and safe antivenom development and distribution represented one of three major factors driving these changes (alongside improved supportive healthcare and expanded healthcare access for underserved communities (Chippaux et al., 2020; National Geographic, 2025; Riaño-Umbarila et al., 2025). These factors have largely circumvented the antivenom cycle, enabling pharmacy-based antivenom purchase without specialized preservation requirements (Riaño-Umbarila et al., 2025; The Eye Mexico, 2025). While this may lead an observer to conclude that the issue of envenomation in Mexico has been solved, that is not the case. Limitations in the healthcare system result in a restricted reach of antivenoms, as isolated, rural, and typically impoverished communities experience disproportionately high scorpion-related mortality (Chippaux & Goyffon, 2008; Abroug et al., 2020). Additionally, while advanced purification methods have significantly reduced adverse effects, some persist. Most importantly, batch-to-batch variability—a fundamental limitation of animal-derived antivenoms—remains. Consequently, two strategies have been implemented by the same research teams responsible for 1990s breakthroughs: (1) using recombinant technology to eliminate toxin extraction from animals by producing toxins in microorganisms, and (2) implementing fully recombinant antivenoms as alternatives to animal-derived products (Riaño-Umbarila et al., 2025; Chippaux et al., 2020; Boyer, 2013; National Geographic, 2025).
