Press the button below to view more information.
Result Preamble
This year’s the iGEM RUM-UPRM R-DetoX team focused on improving the previous project design by reducing the total number of genetic circuits from three — (1) detection, (2) degradation, and (3) kill switch — to two: (1) detection and degradation, and (2) kill switch. This simplification aimed to increase modularity, reduce metabolic burden on the host cells, and improve overall construct stability.

Shown above is the proposed improved genetic construct, represented using SBOL notation. The sequence includes a constitutive promoter, the xplA and xplB genes responsible for RDX degradation, a reporter gene (amilGFP), and a terminator sequence. Additionally, a synthetic riboswitch with an aptamer-based sensor specific to RDX [1] was inserted before each coding sequence to enable ligand-dependent regulation. Based on this design, three genetic circuits were submitted for synthesis, as shown below.



The three genetic circuits shown in Figures 2–4 were submitted for DNA synthesis to Integrated DNA Technologies (IDT). Of these, only one sequence was successfully synthesized and delivered— the control construct shown in Figure 2. This construct was later transformed and cryopreserved for subsequent testing (see Results section).
The other two constructs were not delivered, as the colonies displayed stunted growth during synthesis. The main differences between the successful construct and the ones that failed were their increased sequence length and inclusion of XplA and XplB degradation proteins. We hypothesized that the amount of proteins may have been toxic to the host bacteria or that the longer DNA fragments imposed excessive genetic expression and physiological metabolic stress. Furthermore, the constructs employed a constitutive promoter, which continuously drives transcription; this may have resulted in overexpression of lengthy mRNA transcripts and additional cellular burden, further contributing to the observed synthesis issues.
To address these issues, two hypotheses were proposed: (1) sequence length and continuous transcription imposed excessive metabolic stress, and (2) the degradation proteins were cytotoxic when constitutively expressed. Both challenges could be mitigated by introducing inducible promoters to regulate transcriptional activity.
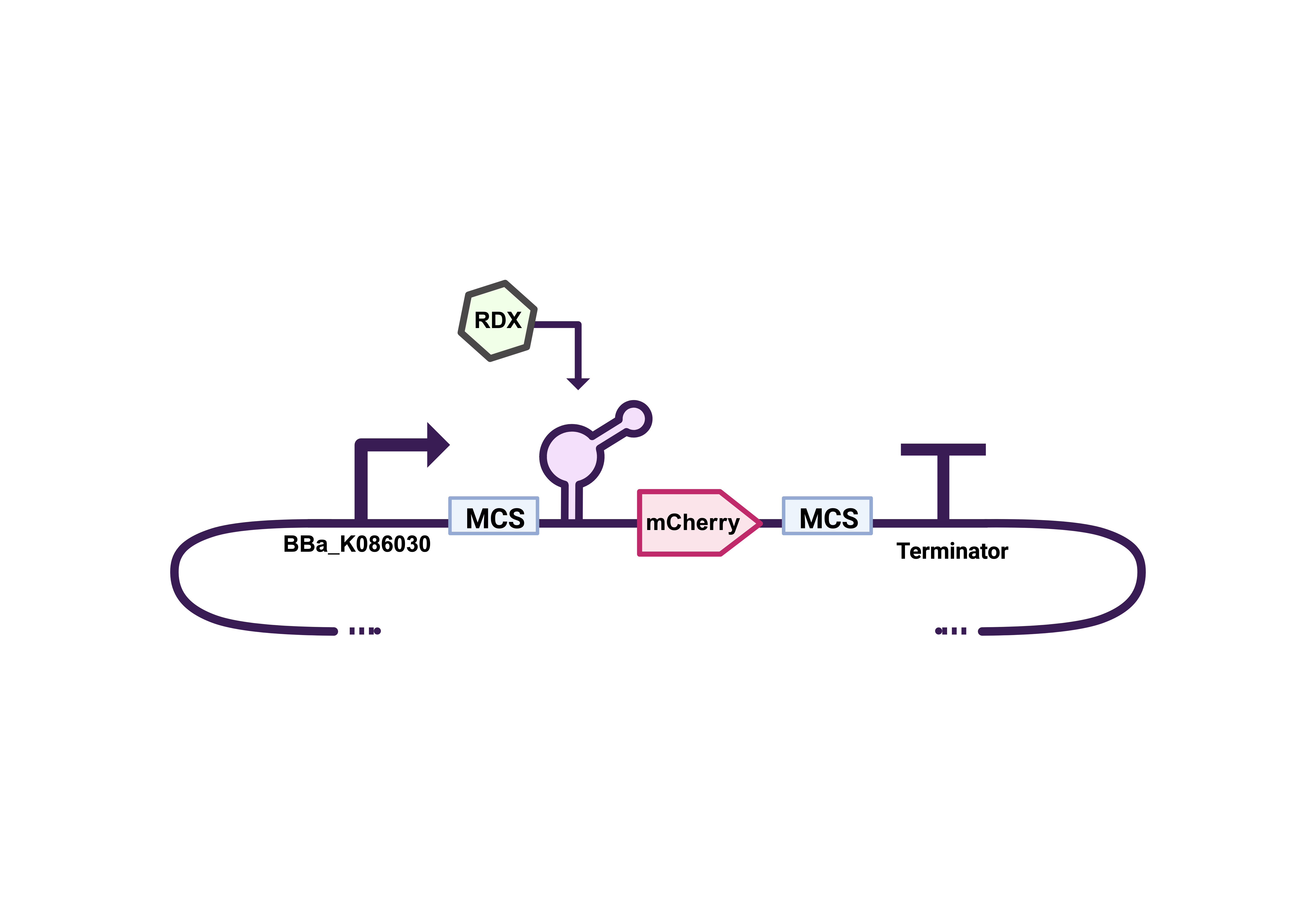
The team identified two stress-inducible promoters from the IIT Madras 2008 iGEM collection: the σ³² Heat Shock promoter (BBa_K086026) and the σ³⁸ Stationary Phase promoter (BBa_K086030) . These allow transcriptional control through environmental conditions—heat shock and stationary-phase signaling, respectively. The σ³⁸ promoter was selected for most laboratory tests due to its IPTG-regulated dual control, while the σ³² promoter was reserved for future bioreactor applications.
Using these promoters, a new modular set of constructs (Figures 5–9) was designed to enable interchangeable regulation between riboswitches, degradation genes, and reporter proteins. These improvements aimed to produce a more stable, controllable, and effective biosensor for RDX detection and degradation.




Results
Even though we only received the prototype shown in Figure 2 (the first order included the sequences of the prototypes shown in Figures 2–4), we decided to conduct several preliminary tests to evaluate the functionality of this device. The vector was first resuspended and successfully transformed into electrocompetent E. coli DH5α cells. The transformed culture was then inoculated in Luria Broth (LB) to perform a DNA extraction for restriction digestion and to prepare cryopreserved stocks for later use. Two restriction digestion reactions were carried out, one using NotI and another using XbaI and SpeI on another, confirming both the expected restriction sites and the correct prototype size. Figure 10 shows the 1% agarose gel electrophoresis in 1X TAE (Tris-acetate-EDTA) buffer of the construct depicted in Figure 2. The first lane contains the 1 kb DNA ladder, followed by the undigested plasmid with prototype DNA, the NotI digestion, and finally the XbaI and SpeI digestion. The results confirmed the expected banding pattern for the construct.


After experiencing some challenges with the synthesis order, likely due to the constitutive promoter overworking the bacteria, we decided to replace it with an inducible promoter. We subsequently identified the σ38 Stationary Phase promoter designed by the IIT Madras 2008 iGEM team. This promoter is activated by the stationary-phase sigma factor and includes Lac operator sites, allowing it to be repressed by LacI unless IPTG is added to the medium. In other words, it is a dual-regulated promoter, responsive to both cellular growth phase and chemical induction. This modification was expected to reduce the stress caused by continuous transcription, potentially resolving the issue of stunted colony growth.
The same iGEM team also developed a σ32 Heat Shock promoter, which we plan to use in future experiments involving bench-scale bioreactor testing. However, for most laboratory assays, the stationary-phase promoter will serve as our primary regulatory element.
With this improvement addressing one potential limitation, we also decided to replace the reporter protein. AmilGFP, a yellow chromoprotein, proved difficult to visualize in LB medium during early qualitative assays, as its signal was barely distinguishable. To improve detection, we substituted it with mCherry, a red fluorescent protein that offers higher contrast with in the medium. With these two design changes implemented, a second synthesis order was placed for the genetic constructs shown in Figures 5–9.
After a few weeks, all synthesis orders were unfortunately canceled except for one, the construct described in Figure 8. Upon receiving this sequence from IDT, it was transformed into electrocompetent E. coli DH5α, cryopreserved with 50% glycerol, and subjected to plasmid extraction using a ZymoPURE II Plasmid Purification Kit. The extracted DNA was then analyzed via restriction digestion to verify the expected vector and insert sizes. The gel electrophoresis results are shown below: the first well contains the NEB 1 kb ladder, followed by the undigested plasmid, and lanes three and four show the NotI and XbaI + SpeI digestions, respectively.


Later, RDX detection tests were conducted using the device shown in Figure 2. This construct contains the riboswitch and amilGFP as the reporter gene, meaning that any detection of RDX could be qualitatively observed through color change. The bacteria were inoculated in LB medium with different RDX concentrations. However, the results were inconclusive because LB is naturally yellow, which interfered with the visualization of the yellow amilGFP signal.
To address this, we switched to colorless media: M9, M9 supplemented with glucose , and RPMI-1640 . Although RPMI-1640 is typically used for eukaryotic cell culture, we tested it due to its translucency. Three concentrations of RDX were evaluated in these media, and analyses were performed in triplicate for M9 with glucose and RPMI-1640.
After 24 hours of incubation, a slight yellow color was observed, suggesting potential RDX detection and amilGFP expression. Quantitative absorbance measurements at 490 nm were performed (Table 1) to validate these preliminary results. The cultures were then incubated for an additional 24 hours to determine whether the signal would intensify. After 48 hours, a stronger yellow color was visible. To better visualize this result, 2 mL bacterial pellets were generated, in which the yellow color was clearly observed. Additional absorbance measurements were also performed to confirm this observation (Table 2). Below are the images of the pellets collected for the colorimetric assay.







After 48 hours of incubation, visible color differences were observed among the bacterial pellets. In nearly all +RDX tests, at least two out of three replicates exhibited a yellow color, except for those cultured in RPMI-1640 medium. Notably, none of the –RDX controls showed any yellow color, confirming that the observed signal was specific to the RDX-treated samples. Pellets from the M9 medium displayed the most pronounced yellow color compared to their controls, suggesting stronger expression of amilGFP. Interestingly, despite this visible intensity, the corresponding absorbance readings were lower than those from RPDMI-1640 and M9+0.2% glucose media.
As shown in the bar charts in Figures 19–21, the absorbance readings for M9 medium were consistently lower than those for M9 + glucose and RPMI-1640. The combination of stronger visual pigmentation but lower absorbance may indicate a lower bacterial growth rate but higher relative reporter protein expression. These results suggest that E. coli grown in the minimal medium without a carbon source may have had greater permeability to RDX, leading to enhanced riboswitch activation and, consequently, increased reporter gene expression.
Another important observation is that the M9 control showed little to no growth, which was expected for inoculations in a minimal medium lacking a carbon source. This outcome could indicate two possibilities: (1) no cells were transferred to the control tube, resulting in no growth, or (2) the presence of RDX in the treated samples may have stimulated or supported limited bacterial growth. In either case, this experiment should be repeated to confirm reproducibility and rule out experimental anomalies. In contrast, the M9 + glucose cultures exhibited weaker yellow coloration, which could be due to reduced RDX permeability caused by the presence of glucose. However, as expected, these samples showed higher overall absorbance values, reflecting greater bacterial growth relative to the other media.
Finally, samples cultured in RPMI-1640 medium displayed no detectable yellow pigmentation across all RDX concentrations. Although this medium is nutrient-rich and optimized for eukaryotic cell culture, it was selected for testing due to its translucency and personal communication of E. coli growth in it. As shown in Figures 12–14, no pigmentation was observed in any replicate. The enriched composition of RPMI-1640 potentially interfered with RDX permeability, preventing riboswitch activation and subsequent reporter expression.
Measurement Charts
| Medium | 1 µM | 2 µM | 3.8 µM |
|---|---|---|---|
| M9 | 1.058 | 1.268 | 0.593 |
| M9 + 0.2% Glucose | N/A | 1.067 | 1.216 |
| 1.397 | 1.271 | 0.465 | |
| 1.360 | 1.414 | 1.279 | |
| RPMI-1640 | 2.398 | 0.854 | 2.584 |
| 0.111 | 0.869 | 2.449 | |
| 0.008 | 2.093 | 1.533 |
| Absorbance Readings at 490 nm; 48 hours | |||
|---|---|---|---|
| Medium | 1 µM | 2 µM | 3.8 µM |
| M9 | 1.699 | 2.243 | 1.384 |
| M9 + 0.2% Glucose | N/A | 2.776 | 3.832 |
| 2.989 | 4.950 | 3.830 | |
| 2.637 | 4.970 | 4.518 | |
| RPMI-1640 | 2.732 | 2.716 | 2.942 |
| 2.487 | 2.578 | 2.857 | |
| 2.590 | 2.931 | 2.615 | |
| Medium | 24 hours | 48 hours |
|---|---|---|
| M9 | 0.019 | 0.013 |
| M9 + 0.2% Glucose | 1.373 | 5.030 |
| RPMI-1640 | 0.182 | 2.467 |
| Medium | RDX (µM) | Time (h) | Rep1 | Rep2 | Rep3 | Control Mean | Mean | SD | SE |
|---|---|---|---|---|---|---|---|---|---|
| M9 + Glucose | 1 | 24 | N/A | 1.067 | 1.216 | 1.373 | 1.142 | 0.105 | 0.075 |
| M9 + Glucose | 1 | 48 | N/A | 2.776 | 3.832 | 5.03 | 3.304 | 0.746 | 0.528 |
| M9 + Glucose | 2 | 24 | 1.067 | 1.271 | 1.414 | 1.373 | 1.251 | 0.174 | 0.101 |
| M9 + Glucose | 2 | 48 | 2.776 | 4.95 | 4.97 | 5.03 | 4.232 | 1.261 | 0.728 |
| M9 + Glucose | 3.8 | 24 | 1.216 | 0.465 | 1.279 | 1.373 | 0.987 | 0.453 | 0.261 |
| M9 + Glucose | 3.8 | 48 | 3.832 | 3.83 | 4.518 | 5.03 | 4.06 | 0.397 | 0.229 |
| Medium | RDX (µM) | Time (h) | Rep1 | Rep2 | Rep3 | Control Mean | Mean | SD | SE |
|---|---|---|---|---|---|---|---|---|---|
| RPMI-1640 | 1 | 24 | 2.398 | 0.111 | 0.008 | 0.182 | 0.839 | 1.351 | 0.780 |
| RPMI-1640 | 1 | 48 | 2.732 | 2.487 | 2.59 | 2.467 | 2.603 | 0.123 | 0.071 |
| RPMI-1640 | 2 | 24 | 0.854 | 0.869 | 2.093 | 0.182 | 1.272 | 0.711 | 0.411 |
| RPMI-1640 | 2 | 48 | 2.716 | 2.578 | 2.931 | 2.467 | 2.742 | 0.178 | 0.103 |
| RPMI-1640 | 3.8 | 24 | 2.584 | 2.449 | 1.533 | 0.182 | 2.189 | 0.572 | 0.332 |
| RPMI-1640 | 3.8 | 48 | 2.942 | 2.857 | 2.615 | 2.467 | 2.805 | 0.170 | 0.098 |



The most interesting results were obtained from the M9 medium without glucose. These samples were originally expected to show no bacterial growth, as no carbon source was provided—consistent with the M9 control, which showed no growth. Surprisingly, however, E. coli inoculated in M9 medium containing RDX not only grew but also exhibited the most intense yellow pigmentation among all treatments. This observation suggests that the cells may have utilized RDX or possibly the acetone used to dissolve it as an alternative carbon source.
In future experiments, this assay should be repeated with a higher number of replicates for both control and experimental groups to corroborate these findings. If reproducible, subsequent analyses should investigate whether E. coli is capable of degrading RDX in the absence of xplA and xplB, which could indicate previously unreported metabolic activity. Confirming this possibility would have significant implications for the future direction of the project and the design of the biosensor system.
Overall, these findings suggest that the biosensor construct remains potentially responsive to RDX exposure, particularly under minimal medium conditions where membrane permeability may be enhanced. Nonetheless, further quantitative validation and repeated trials are required to confirm these preliminary results and assess the reproducibility and sensitivity of the system.
Ongoing:
As previously mentioned, the second synthesis order presented a challenge, as only one out of the six ordered constructs were successfully delivered. Upon closer inspection of the submitted sequences, we identified that in both control constructs (Figures 5 and 6), a termination sequence had been mistakenly placed immediately after the promoter. This likely explains why both constructs encountered issues during transformation.
Additionally, we noticed that the selected vector was not ideal for our construct design. Up to this point, all sequences had been ordered using an expression vector (pET-IDT) that already included its own promoter, ribosome binding site (RBS), and terminator elements surrounding the synthesized insert. This configuration may have interfered with proper transcription of our designed sequences, contributing to the synthesis and transformation difficulties reported by IDT.
After identifying these issues, the incorrect placement of the termination sequence in both control constructs was corrected, and a third synthesis order was placed for the constructs represented in Figures 5, 6, 7, and 9, this time using IDT’s pUCIDT (Kan) vector, a much better fit for our genetic devices. As of this writing (Sunday, October 5, 2025), the order has not yet arrived; however, the team has received confirmation from an IDT representative that “the lab has identified perfect colonies for all four genes in the new order.” We expect the sequences to arrive by October 14, 2025. Although we will not be able to test these constructs before the 2025 Grand Jamboree, we plan to continue developing and characterizing them after this year’s competition cycle concludes.
References
[1] Eberly, J. O., Mayo, M. L., Carr, M. R., Crocker, F. H., & Indest, K. J. (2019). Detection of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) with a microbial sensor. Journal of General and Applied Microbiology, Advance Publication. https://doi.org/10.2323/jgam.2018.08.001
[2] Mayo, M. L., Eberly, J. O., Crocker, F. H., & Indest, K. J. (2020). Modeling a synthetic aptamer-based riboswitch biosensor sensitive to low hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) concentrations. PLOS ONE, 15(11), e0241664. https://doi.org/10.1371/journal.pone.0241664
[3] iGEM Foundation. (n.d.). Registry of Standard Biological Parts. Retrieved October 5, 2025, from https://parts.igem.org/Main_Page
