Abstract
Precise control of protein activity is fundamental to cell engineering and underpins the development of advanced cell-based therapies. While transcriptional and translational regulation provide powerful means of control in mammalian cells, post-translational regulation offers distinct advantages by enabling immediate and reversible responses to external stimuli. Here, we present SPARK (Signal-Programmable Activation of Regulated seCretion), which employs engineered molecular anchors to tether secretory vesicles carrying the target protein to the actin cytoskeleton and release them upon signal induction through protein dissociation or cleavage. We demonstrated that SPARK enabled efficient light- and chemically induced secretion of reporter proteins in HEK293T cells, exhibiting a markedly faster response than traditional transcriptional control systems. This platform provides rapid, tunable, and precise control of protein secretion, establishing a versatile foundation for on-demand therapeutic protein production and other applications that require fast and programmable protein release.
Introduction
Precise control of cellular protein activity is a central goal in synthetic biology and underpins the development of programmable therapeutic cells. By constructing genetic circuits that can sense user-defined signals and respond through controlled expression of therapeutic proteins, researchers have created new possibilities for dynamic disease intervention1.
Over the past decade, a variety of stimulus-responsive cellular systems have been developed. Some respond to external physical cues, such as electromagnetic fields2 or blue light, while others react to internal physiological signals, such as blood glucose3 or pH4. These systems typically rely on transcriptional or translational regulation to control target gene expression, allowing engineered cells to detect and respond to disease states such as obesity5, type 2 diabetes6, and cancer7.
However, our literature review and expert consultation (see our Human Practices page) revealed a key limitation that hinders clinical translation. Most existing gene switches operate at the transcriptional8 or translational9 level, requiring hours for therapeutic proteins to be synthesized. Such delays can greatly limit their utility in certain therapeutic scenarios, such as insulin secretion for type 1 diabetes (T1D), and prevent timely physiological intervention.
To overcome this limitation, we sought to design a rapid, post-translational regulatory system that allows immediate and reversible control of protein secretion in mammalian cells. We developed SPARK (Signal-Programmable Activation of Regulated seCretion), a synthetic platform that couples signal perception to fast protein release. SPARK integrates three key modules: a Secretory module that directs the target protein into secretory vesicles, an Anchor module that tethers these vesicles to the actin cytoskeleton for stable intracellular retention, and a Signal-responsive module that releases the vesicles upon specific signal induction.
To demonstrate the versatility of this system, we engineered two variants—optoSPARK and chemSPARK, tailored for different input modalities. The optoSPARK system incorporates photoreactive protein modules responsive to distinct wavelengths of light, including violet (mMaple3), green (CarH), and near-infrared (BphP1-PpsR2). Upon illumination, these modules undergo conformational dissociation or cleavage, releasing the tethered secretory vesicles and inducing rapid protein secretion. Significant increases in secreted protein levels were detected within two hours of optical stimulation.
The chemSPARK system utilizes the NS3-Binder interaction pair from the hepatitis C virus10,11. In the presence of the FDA-approved drug grazoprevir, the NS3–Binder complex dissociates specifically, triggering immediate release of the target protein. Elevated secretion was observed within two hours of chemical induction.
By combining vesicular retention, cytoskeletal anchoring, and signal-responsive release modules, SPARK establishes a new post-translational regulatory paradigm for achieving fast and controllable protein secretion in mammalian cells. This system provides a versatile foundation for on-demand therapeutic protein production and other applications requiring precise, rapid, and programmable protein control.
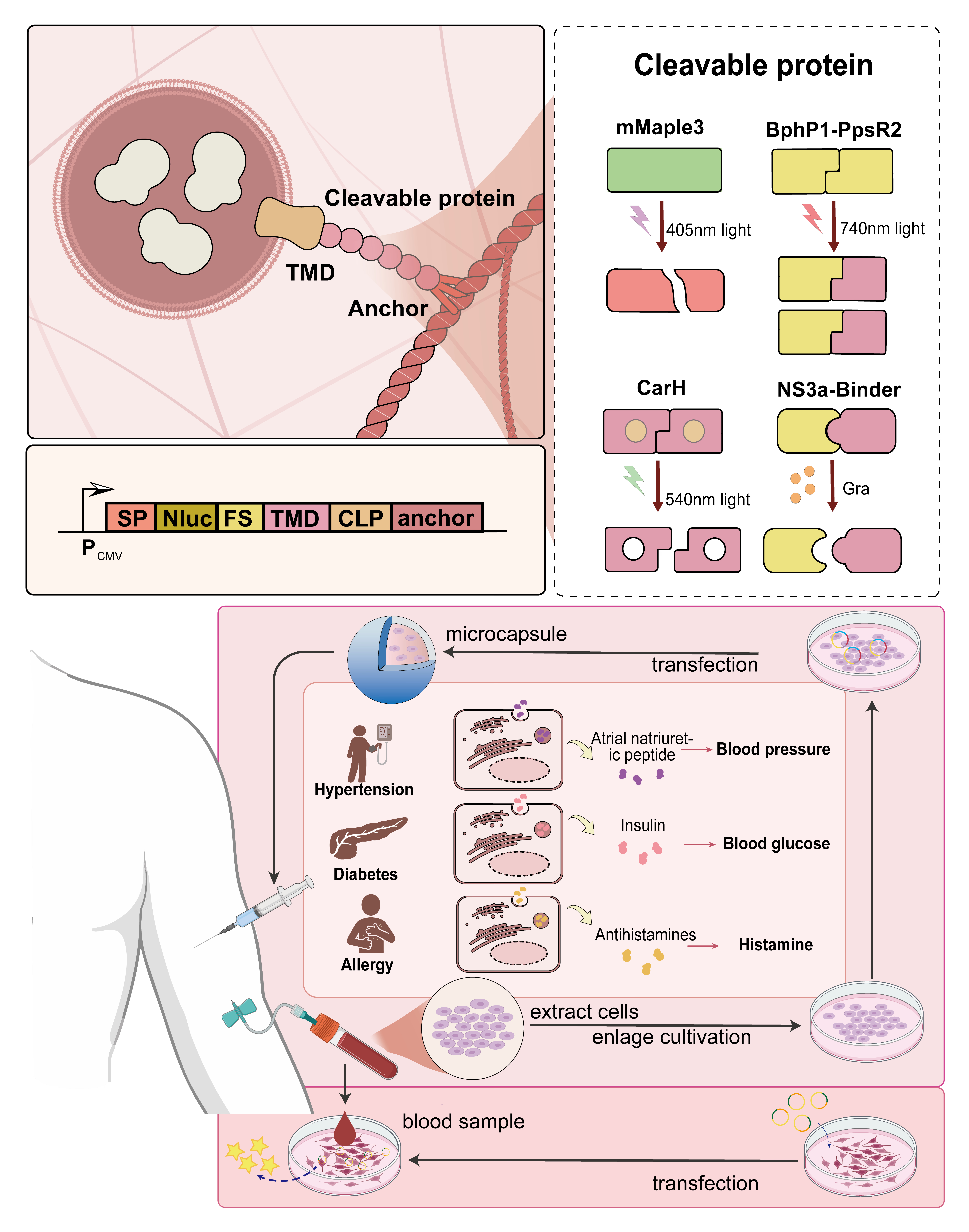
Figure 1 Graphical Description of SPARK
 Description
Description
 Design
Design
 Notebook
Notebook
 Results
Results
 Basic Parts
Basic Parts
 Composite Parts
Composite Parts
 Parts Collection
Parts Collection
 Education
Education
 Art Gallery
Art Gallery
 Implementation
Implementation
 Attributions
Attributions
 Collaboration
Collaboration