Introduction
Our goal was to engineer a signal-controlled protein secretion switch in mammalian cells. Following multiple Design-Build-Test-Learn (DBTL) cycles (Figure 1), we developed two modular systems:
- OptoSPARK - light-inducible protein secretion
- ChemSPARK - small-molecule-inducible protein secretion
Both switches regulate secretion post-translationally, enabling responses that are faster and more sensitive than conventional transcriptional control. Below, we detail the iterative DBTL cycles that led from vesicle anchoring to light and drug control, then to performance optimization and benchmarking.

Figure 1. A schematic Diagram of the Engineering process (you can scroll horizontally to see more information).
Stage I: Secretion switch based on Photolytic protein
Secretion switch foundation: vesicle anchoring with LifeAct
Design:
To block constitutive secretion and create a controllable "release" step, we first needed to retain secretory vesicles carrying the protein of interest (POI). We fused the POI to a furin site-containing transmembrane domain (TMD) so the cargo is released into the vesicle lumen after Golgi trafficking, and then added LifeAct, a 17-aa peptide that binds F-actin with high specificity 1,2. The design tethers vesicles to the actin cytoskeleton (Figure 2). NanoLuc (Nluc), fused to an IgK signal peptide, served as a quantitative secreted reporter.
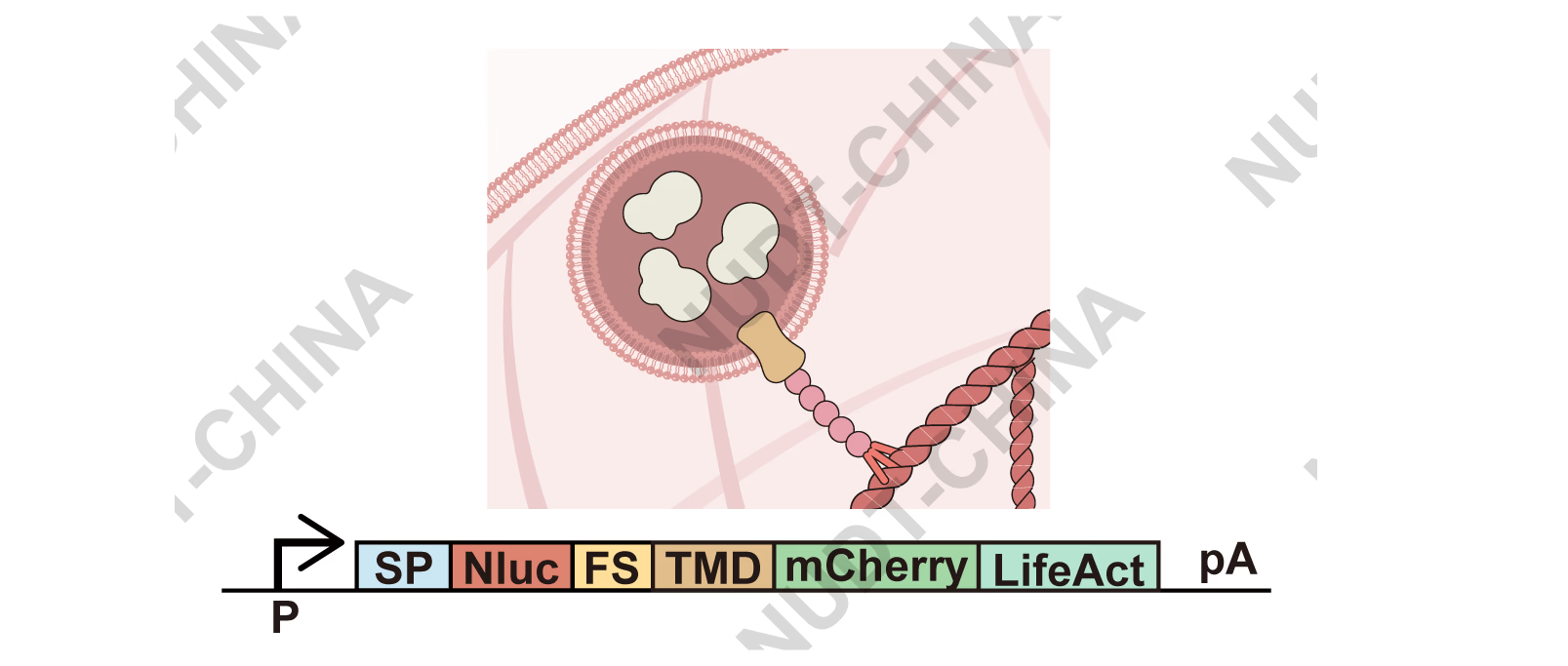
Figure 2. Schematic of the vesicle-anchoring module.
Build & Test:
HEK293T cells were transfected with pNC25038 (PCMV-IgK-Nluc-FS-TMD-LifeAct) or pNC25039 (PCMV-IgK-Nluc-FS-TMD-mCherry). Results showed that cells expressing pNC25038 showed a significant decrease in NanoLuc secretion compared with controls (Figure 3A), confirming effective vesicle retention. Electron microscopy of cells expressing pNC25085 (EGFP-TMD-LifeAct) showed vesicle accumulation intracellularly (Figure 3B). Similarly, live-cell fluorescence imaging also demonstrated clear co-localization of EGFP-TMD-LifeAct with mCherry-LifeAct along filamentous actin (Figure 4), confirming that vesicles were immobilized on the cytoskeleton.
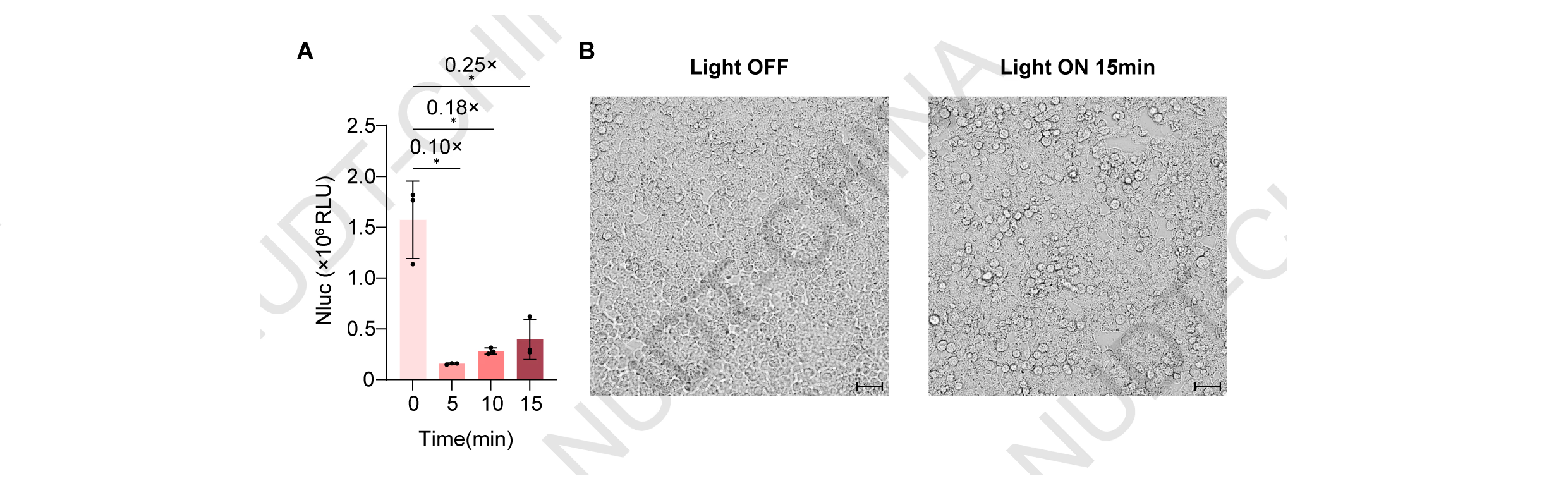
Figure 3. LifeAct fusion enables robust retention of secretory vesicles in mammalian cells
(A) Supernatant NanoLuc levels in HEK293T cells transfected with either pNC25038 (PCMV-IgK-Nluc-FS-TMD-LifeAct) or pNC25039 (PCMV-IgK-Nluc-FS-TMD-mCherry). NanoLuc activity was measured 48 h post-transfection. Data represent mean ± SD from n = 4 independent experiments; ***P<0.001, two-tailed unpaired t-test.
(B) Representative transmission electron microscopy images of HEK293T cells expressing POI-TMD-LifeAct, showing vesicle accumulation near the actin cytoskeleton. Samples were collected 48 hours post transfection. Scale bars, 2 μm (left) and 1 μm (right).

Figure 4. Confocal micrograph of fluorescence protein localization.
Representative live-cell fluorescence imaging of HEK293T cells co-transfected with pNC25086 (PCMV-LifeAct-mCherry) and either pNC25087 (PCMV-EGFP-TMD; upper panels) or pNC25085 (PCMV-EGFP-TMD-LifeAct; lower panels). Fluorescent images were taken 24 hours post transfection Scale bars, 10 μm (upper) and 10 μm (lower).
Learn:
LifeAct reliably terminates constitutive secretion by anchoring vesicles, providing a solid foundation for signal-induced release in subsequent cycles.
OptoSPARK V0.5: photolytic release (mMaple3)
Design:
We next tested whether light-induced disruption of the anchoring complex could trigger secretion. We built POI-TMD-mMaple3-LifeAct, leveraging mMaple3, a photoconvertible FP that turns green-to-red upon 405 nm illumination and undergoes a cleavage-like structural change near aa 66-67 3,4, expected to release anchored vesicles (Figure 5).
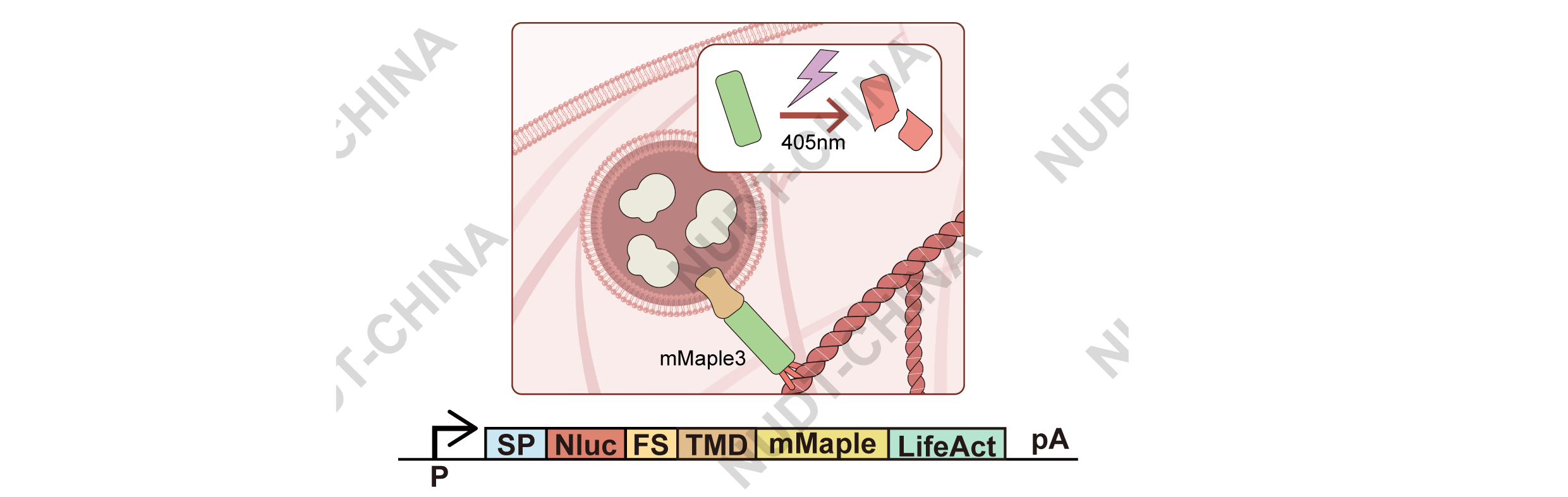
Figure 5. Schematic diagram illustrating the operation of the secretion system genetic switch in HEK-293T cells under violet light induction
Build & Test:
HEK293T cells were transfected with pNC25088 (PCMV-IgK-Nluc-FS-TMD-mMaple3-LifeAct) and illuminated using our custom 405 nm light device (10 s ON / 15 s OFF, 10 min). Illumination induced strong red fluorescence, confirming mMaple3 photoconversion (Figure 6A), and a modest but reproducible increase in NanoLuc secretion compared with dark controls (Figure 6B). However, control cells expressing pNC25089 (PCMV-IgK-Nluc) showed a strong reduction in NanoLuc activity and visible stress under the same illumination, indicating that violet-light exposure was cytotoxic (Figure 6C-D).
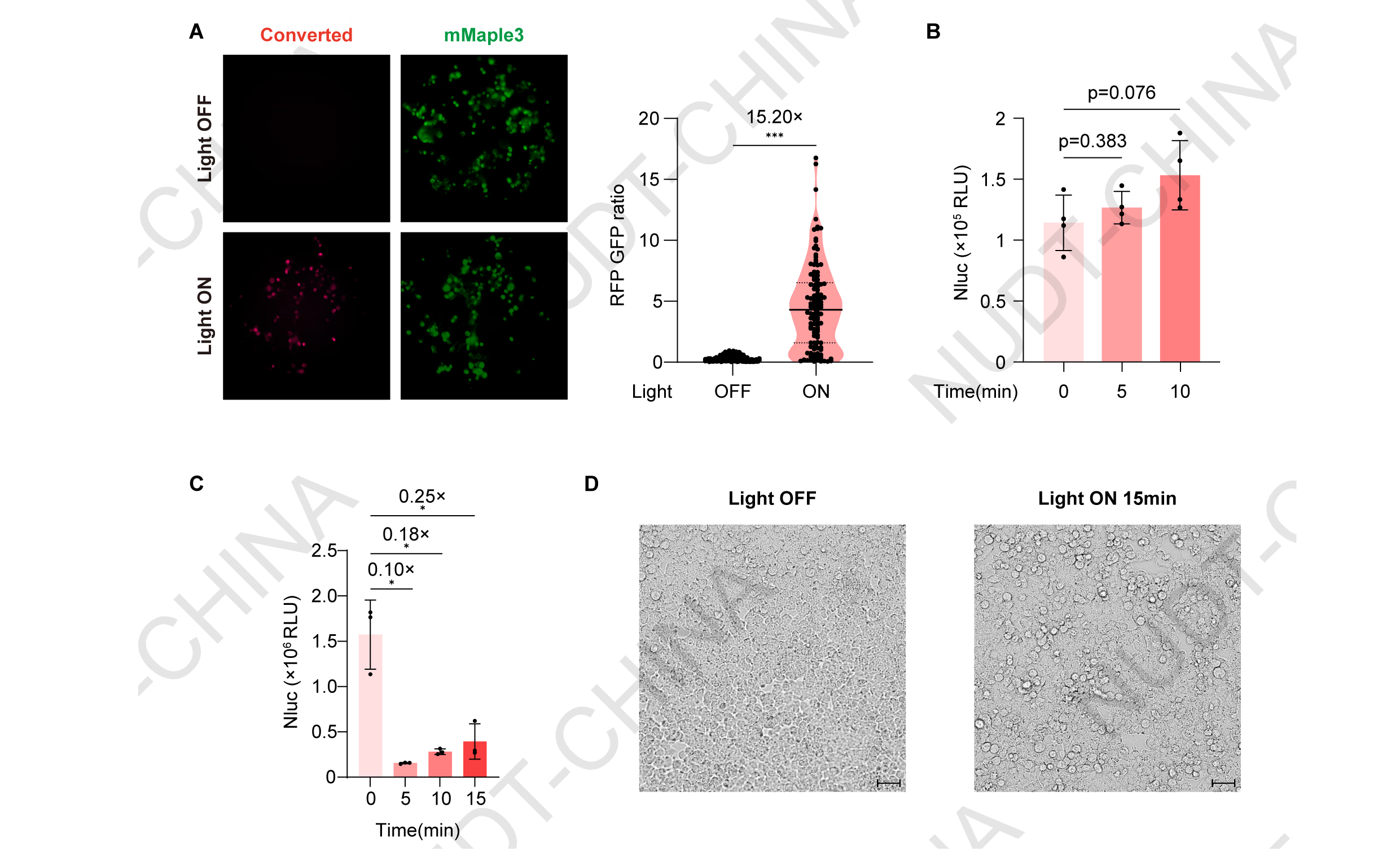
Figure 6. Engineering of Secretion switch based on mMaple Photolytic protein.
(A) Cellular Red Fluorescence / Green Fluorescence intensity. Representative fluorescence images of pNC25088 (PCMV-IgK-Nluc-FS-TMD-mMaple3-LifeAct) HEK293T cells maintained in the dark (control) or stimulated with violet light (Light). Fluorescent images were taken 48 hours post transfection. Scale bar, 50 µm. Quantification of single-cell red/green fluorescence ratios was performed using ImageJ. ***P<0.001, two-tailed unpaired t-test.
(B) NanoLuc secretion levels under different stimulation durations. HEK293T cells were transfected with pNC25088 (PCMV-IgK-Nluc-FS-TMD-mMaple3-LifeAct). 48 hours post-transfection, cells were illuminated with pulsed violet light (405 nm; 10 s ON/15 s OFF). Supernatants were collected before stimulation (0 min) and at 5 and 10 min after light exposure to quantify NanoLuc activity. Data represent mean ± SD from n = 4 independent experiments; p-value obtained by two-tailed unpaired t-test.
(C) Effect of violet-light stimulation on constitutive protein secretion. HEK293T cells transfected with pNC25089 (PCMV-IgK-Nluc) were illuminated with pulsed violet light (405 nm; 10 s ON/15 s OFF) 48 h post-transfection. NanoLuc activity in culture supernatants was measured before stimulation at 5, 10, and 15min after illumination. Data represent mean ± SD from n = 4 independent experiments.
(D) Representative phase-contrast images of HEK293T cells transfected with pNC25088 (PCMV-lgK-Nluc-FS-TMD-mMaple3-LifeAct) maintained in the dark or after violet-light stimulation (405 nm; 10 s ON/15 s OFF) for 15 min. Scale bar, 50 µm.
Learn:
The experiment validated the concept of light-induced vesicle release but revealed that violet light causes significant cytotoxicity. Building on these results and the input we obtained from human practice activities (see our Human Practices page for more details), we decided to pursue longer-wavelength, dissociation-based mechanisms for improved biocompatibility.
Stage II: Secretion switch based on signal-inducible protein dissociation
OptoSPARK V1.0: long-wavelength photodissociation (CarH and BphP1-PpsR2)
Design:
Inspired by feedback from our Human Practices discussions, we aimed to design a system responsive to longer wavelengths of light, which are safer for cells and penetrate tissue more effectively. Also, we decided to focus on light-induced protein dissociation rather than photolysis.
Two photoreactive protein pairs were selected:
CarH (green light, 540 nm): oligomerizes in the dark with vitamin B₁₂ but dissociates upon illumination5.
BphP1-PpsR2 (near-infrared light, 740 nm): dissociates upon photoconversion of BphP1 from the Pfr to the Pr state6.
In both systems, dissociation breaks the LifeAct anchoring, triggering vesicle release (Figure 7).
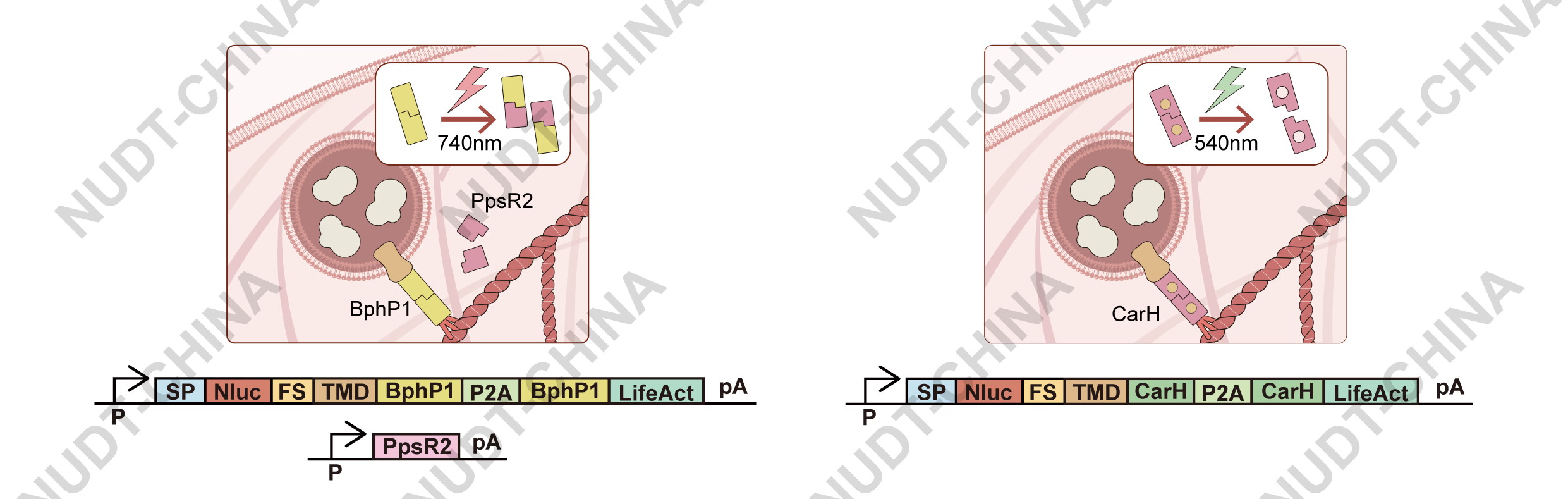
Figure 7. Schematic illustration of the near-infrared (NIR)-responsive secretion switch and the green-light-responsive secretion switch.
Build & Test:
- NIR-light system: Cells were co-transfected with pNC25054 (PCMV-PpsR2), pNC25056 (PCMV-BphP1-LifeAct), and pNC25057 (PCMV-IgK-Nluc-FS-TMD-BphP1) in a 1:1:1 ratio and illuminated with 740 nm light (30 s ON / 210 s OFF, 2 h), producing similar light-dependent secretion increases (Figure 8A).
- Green-light system: HEK293T cells were transfected with pNC25041 (PCMV-IgK-Nluc-FS-TMD-2×GS-CarH) and pNC25043 (PCMV-LifeAct-CarH) in a 1:6 ratio. Cells illuminated with 540 nm light (30 s ON / 30 s OFF, 2 h) showed increased NanoLuc secretion compared with dark controls (Figure 8B).
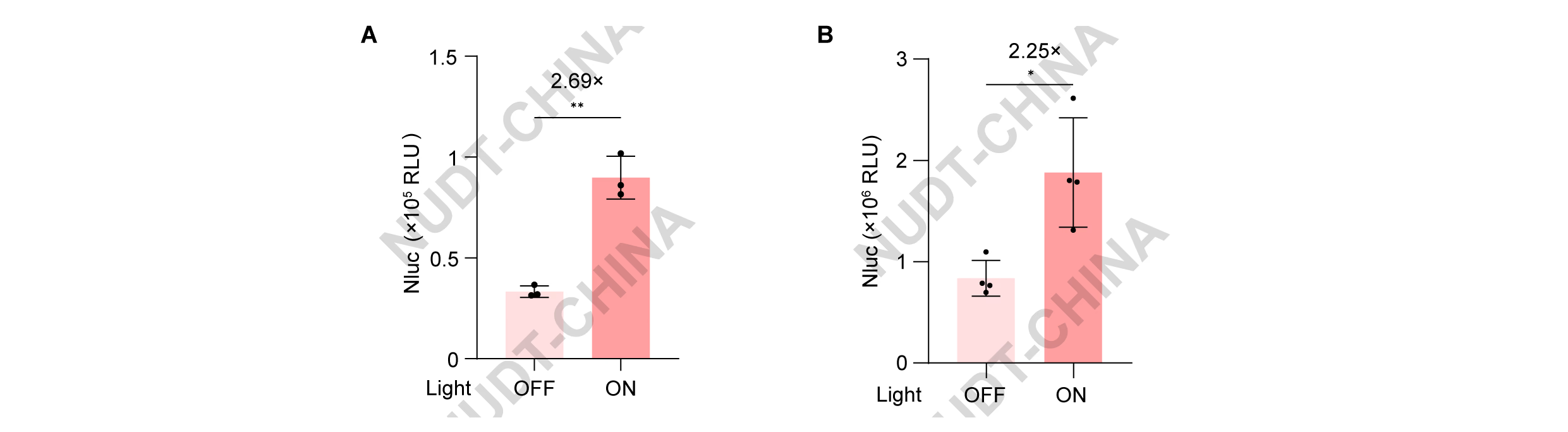
Figure 8. Characterization experiments of the two types of switches based on Photosynthetic dissociation protein.
(A) NanoLuc secretion of HEK293T cells transfected with pNC25054 (PCMV-PpsR2), pNC25056 (PCMV-BphP1-LifeAct), and pNC25057 (PCMV-IgK-Nluc-FS-TMD-BphP1) in a 1:1:1 ratio. Cells were illuminated with pulsed NIR light (740 nm; 30 s ON / 210 s OFF) 48 hours post transfection, NanoLuc activity was measured immediately after illumination. Data represent mean ± SD from n = 4 independent experiments. ** 0.001<P<0.01, two-tailed unpaired t-test.
(B) NanoLuc secretion of HEK293T cells transfected with pNC25041 (PCMV-IgK-Nluc-FS-TMD-2×GSlinker-CarH) and pNC25043 (PCMV-LifeAct-CarH) in a 1:6 ratio. Cells were illuminated with pulsed green light (540 nm; 30 s ON / 30 s OFF) 48 hours post transfection, NanoLuc activity was measured immediately after illumination. Data represent mean ± SD from n = 4 independent experiments. * 0.01<P<0.05, two-tailed unpaired t-test.
Learn:
OptoSPARK V1.0 demonstrated effective secretion under low-energy light without detectable cytotoxicity. This confirmed that light-induced protein dissociation is a viable strategy for biocompatible, optical control of protein secretion. However, we also observed that homodimerization of anchor proteins could reduce retention efficiency, leading us to develop a chemically inducible version of the switch.
ChemSPARK V1.0: chemically induced dissociation (Binder/NS3a)
Design:
To expand SPARK into a clinically relevant platform, we engineered a small-molecule-responsive switch using the interaction between Binder (Apo-NS3a reader) and the HCV protease NS3aH1. These proteins form a stable complex that can be rapidly dissociated by grazoprevir, an FDA-approved NS3a inhibitor7.
In this system (Figure 9), NS3a was fused to the TMD (vesicle side) and Binder to LifeAct (anchoring side). Grazoprevir treatment disrupts the Binder-NS3a complex, releasing vesicles and inducing secretion.
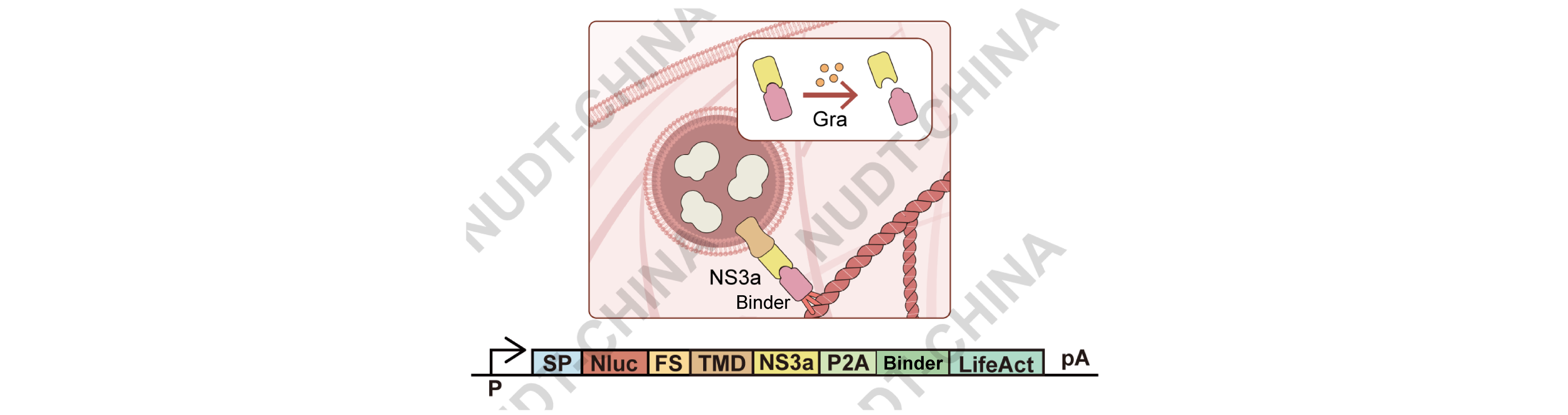
Figure 9. Schematic diagram of the secretion switch design that based on the Grazoprevir-induced disassociation between NS3a and Binder.
Build & Test:
HEK293T cells co-transfected with pNC25091 (PCMV-Binder-LifeAct) and pNC25090 (PCMV-Nluc-TMD-NS3a) were treated with DMSO or grazoprevir. Grazoprevir induced a mild but consistent increase in NanoLuc secretion compared with controls (Figure 10).
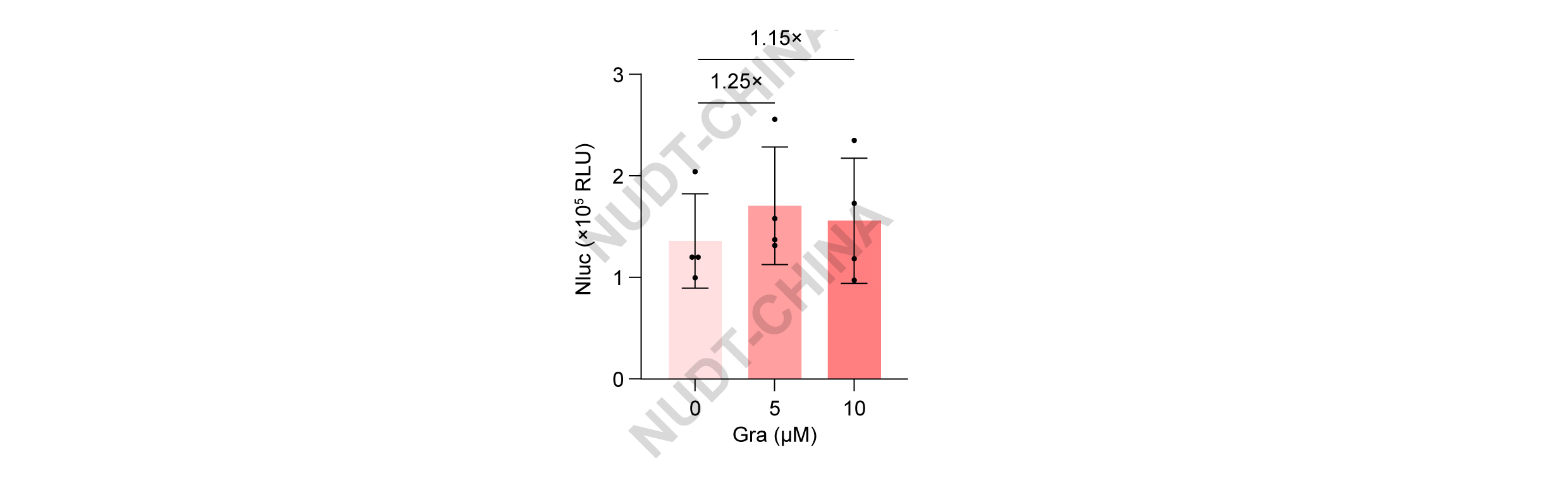
Figure 10. The construction of the secretion switch based on chemical signal-induced protein disassociation in HEK-293T cells.
NanoLuc secretion of HEK293T cells co-transfected with pNC25091 (PCMV-Binder-LifeAct) and pNC25090 (PCMV-Nluc-TMD-NS3a). Cells were treated with DMSO or grazoprevir at the indicated concentrations 48 hours post transfection, Nanoluc levels were quantified 2 hours post induction. Data represent mean ± SD from n = 4 independent experiments measured 2 h after stimulation. Data represent mean ± SD from n = 4 independent experiments.
Learn:
ChemSPARK achieved drug-induced secretion, confirming the feasibility of chemical control. However, the initial response amplitude was modest, motivating further optimization.
ChemSPARK V2.0: optimization and benchmarking
Design:
To enhance chemSPARK's performance, we optimized two key parameters (Figure 11):
- Copy numbers of Nluc and NS3a within expression constructs
- Transfection ratio between the anchoring (Binder-LifeAct) and secretion (Nluc-TMD-NS3a) modules
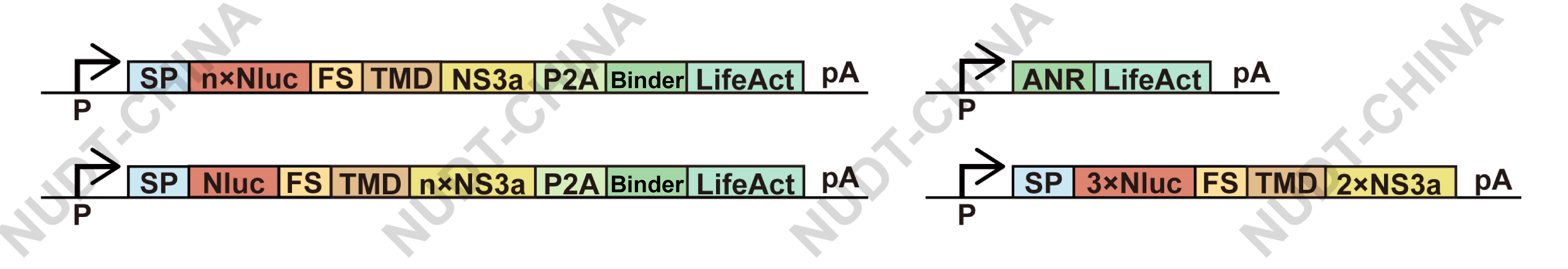
Figure 11. The gene circuit of chemSPARK.
Build & Test:
- Copy number optimization: Increasing Nluc copy number enhanced total secretion, while increasing NS3a reduced basal secretion, improving fold-change response (Figure 12A).
- Transfection ratio optimization: A 3:1 ratio of secretion to anchoring modules provided the best balance between expression strength and responsiveness (Figure 12B).
- Dose dependence: NanoLuc secretion increased up to 40.1× in a grazoprevir dose-dependent manner (0-10 µM, 2 h post-induction; Figure 12C).
- Kinetic comparison: ChemSPARK responded in less than an hour, whereas a transcriptional control system (pNC25046, pNC25048, pNC25098) required >12 hours to produce detectable secretion (Figure 12D).

Figure 12. The characterization of the secretion switch based on chemical signal-induced protein disassociation in HEK-293T cells.
(A) Effect of varying Nluc and NS3a copy numbers on secretion efficiency. HEK293T cells were transfected with either pNC25093 (PCMV-IgK-Nluc-TMD-NS3a-Binder-LifeAct), pNC25094 (PCMV-IgK-(Nluc)2-TMD-NS3a-Binder-LifeAct), pNC25095 (PCMV-IgK-(Nluc)3-TMD-NS3a-Binder-LifeAct), pNC25096 (PCMV-IgK-Nluc-TMD-(NS3a)2-Binder-LifeAct), or pNC25097 (PCMV-IgK-Nluc-TMD-(NS3a)3-Binder-LifeAct). Cells were treated with DMSO or grazoprevir 48 h post-transfection, and NanoLuc levels were quantified 2 h after stimulation. Data represent mean ± SD from n = 4 independent experiments. ** 0.001<P<0.01, two-tailed unpaired t-test.
(B) Optimization of transfection ratio between anchoring and secretion modules. HEK293T cells were co-transfected with pNC25092 (PCMV-IgK-(Nluc)3-TMD-(NS3a)2) and either pNC25086 (PCMV-LifeAct-mCherry; control) or pNC25091 (PCMV-Binder-LifeAct). Cells were treated with DMSO or grazoprevir 48 h post-transfection, and NanoLuc levels were quantified 2 h after stimulation. Data represent mean ± SD from n = 4 independent experiments. Data represent mean ± SD from n = 4 independent experiments. ** 0.001<P<0.01, two-tailed unpaired t-test.
(C) Dose-dependent secretion response to Grazoprevir treatment (0–10 µM). Data presents mean ±SD, n = 3 independent experiments, fitted to a symmetric sigmoid curve.
(D) Kinetics of grazoprevir-inducible NanoLuc expression comparing chemSPARK with a transcriptional activation system. For the transcriptional system, HEK293T cells were co-transfected with pNC25046 (PCMV-Binder-TetR-NLS-VP64), pNC25048 (PCMV-IgK-Fluc-TMD-NS3a), and pNC25098 (PTCE-IgK-Nluc). For the secretion system, cells were co-transfected with pNC25092 (PCMV-(Nluc-FS)3-TMD-(NS3a)2) and pNC25091 (PCMV-LifeAct-mCherry-Binder). In both systems, 10 µM grazoprevir was added at the indicated time points (6 h post-transfection for transcriptional and 48 h for secretion), and NanoLuc levels were monitored over the following 48 h and 12 h, respectively. Data represent mean ± SD of fold changes calculated as the ratio of NanoLuc levels in grazoprevir-treated versus untreated samples (n = 3 independent experiments).
Learn:
ChemSPARK achieved rapid, tunable, and dose-dependent secretion with activation kinetics far exceeding transcriptional regulation.
Through Human Practices, Prof. Jiawei Shao (Zhejiang University School of Medicine) suggested future development of closed-loop, endogenous signal-responsive systems (e.g., calcium-responsive secretion) to enable autonomous physiological regulation---an exciting next step beyond iGEM.
Conclusion
Through iterative DBTL cycles, we engineered a family of signal-controlled secretion systems in mammalian cells:
- LifeAct anchoring enabled reliable vesicle retention.
- Light-induced photodissociation achieved safe, rapid optical control.
- Drug-induced dissociation produced a clinically compatible, tunable switch.
Together, optoSPARK and chemSPARK demonstrate a robust, modular framework for post-translational regulation of secretion, achieving fast, programmable, and biocompatible control of protein output.
 Description
Description
 Design
Design
 Notebook
Notebook
 Results
Results
 Basic Parts
Basic Parts
 Composite Parts
Composite Parts
 Parts Collection
Parts Collection
 Education
Education
 Art Gallery
Art Gallery
 Implementation
Implementation
 Attributions
Attributions
 Collaboration
Collaboration