LifeAct fusion enables robust retention of secretory vesicles in mammalian cells
Before establishing signal responsiveness within the constitutive secretion pathway, we first needed to prevent secretory vesicles containing the protein of interest (POI) from being released constitutively. To achieve this, the POI was fused to a furin site-containing transmembrane domain (TMD), which permits proteolytic release of the cargo into the vesicle lumen following trafficking through the Golgi apparatus. The TMD was further fused to LifeAct, a 17-amino acid peptide that binds F-actin with high specificity1, enabling vesicles carrying the POI to tether to the actin cytoskeleton (Figure 1A).
To evaluate the anchoring efficiency of LifeAct, HEK293T cells were transfected with either pNC25038 (PCMV-IgK-Nluc-FS-TMD-LifeAct) or pNC25039 (PCMV-IgK-Nluc-FS-TMD-mCherry), where the secreted reporter NanoLuc served as the POI. Notably, cells expressing POI-TMD-LifeAct showed a marked reduction in extracellular NanoLuc activity compared with controls, indicating effective intracellular retention of the reporter (Figure 1B).
To directly visualize vesicle localization, we performed electron microscopy and live-cell fluorescence colocalization analyses. Electron microscopy of cells transfected with pNC25085 (PCMV-EGFP-TMD-LifeAct) revealed abundant vesicular structures accumulated near the actin cytoskeleton, consistent with vesicle retention (Figure 1C). Consistent to these findings, cells co-transfected with pNC25086 (PCMV-LifeAct-mCherry) and pNC25087 (PCMV-EGFP-TMD) showed green fluorescence distributed on cell membrane and secretory pathway, reflecting normal vesicle mobility and secretion. In contrast, cells co-transfected with pNC25086 and pNC25085 exhibited strong colocalization of green and red fluorescence along filamentous actin, indicating that vesicles were immobilized on the cytoskeleton (Figure 1D).
Together, these results demonstrate that LifeAct functions as a robust anchoring domain that effectively retains secretory vesicles in mammalian cells, thereby establishing a foundation for subsequent signal-responsive secretion control.
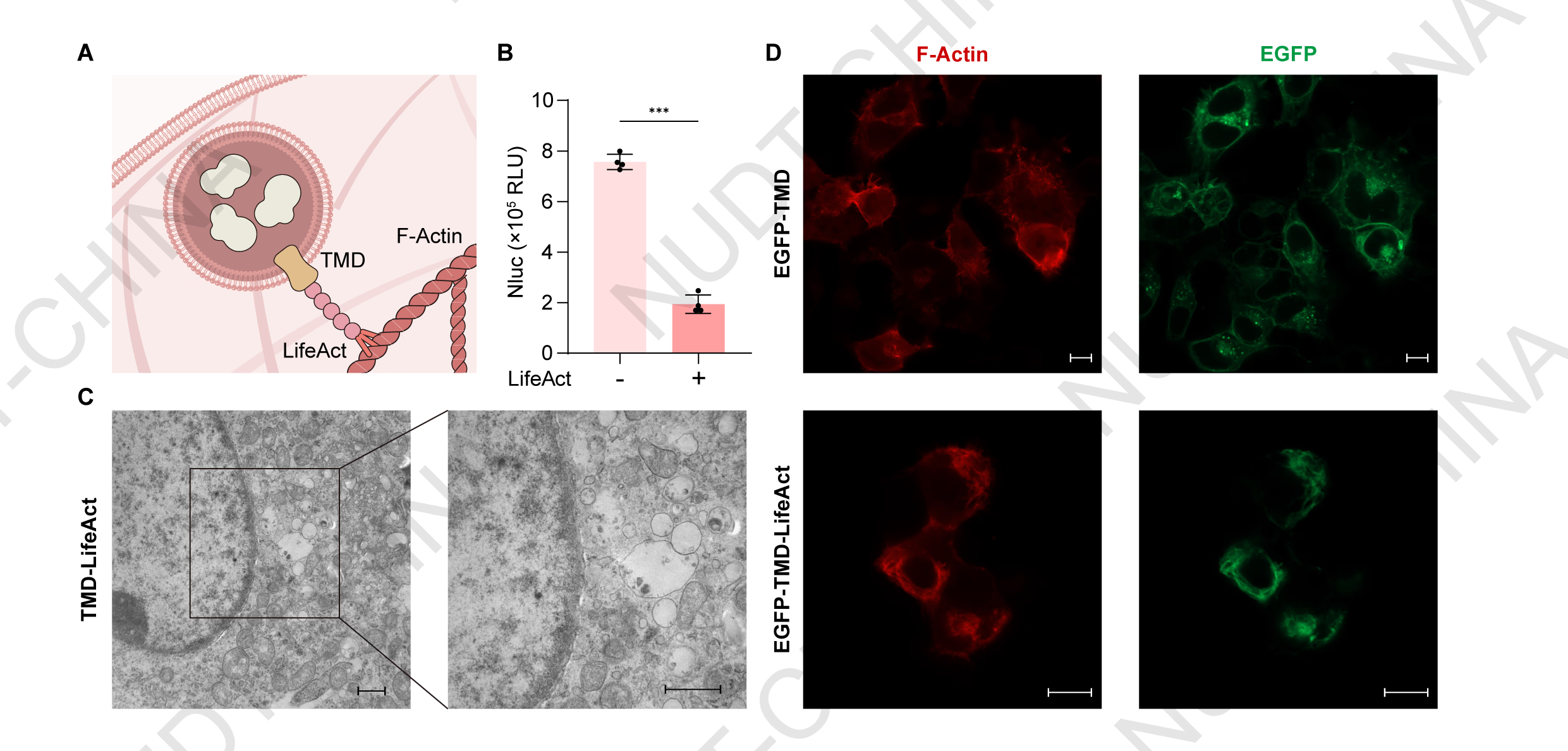
Figure 1.LifeAct fusion enables robust retention of secretory vesicles in mammalian cells.
(A) Schematic illustration of the retention design. LifeAct functions as an anchoring peptide that tethers secretory vesicles to the actin cytoskeleton, preventing constitutive release.
(B) Supernatant NanoLuc levels in HEK293T cells transfected with either pNC25038 (PCMV-IgK-Nluc-FS-TMD-mCherry-LifeAct) or pNC25039 (PCMV-IgK-Nluc-FS-TMD-mCherry). NanoLuc activity was measured 48 h post-transfection. Data represent mean ± SD from n = 4 independent experiments; ***P < 0.001, two-tailed unpaired t-test.
(C) Representative transmission electron microscopy images of HEK293T cells expressing POI-TMD-LifeAct, showing vesicle accumulation near the actin cytoskeleton. Samples were collected 48 hours post transfection. Scale bars, 2 μm (left) and 1 μm (right).
(D) Representative live-cell fluorescence imaging of HEK293T cells co-transfected with pNC25086 (PCMV-LifeAct-mCherry) and either pNC25087 (PCMV-EGFP-TMD; upper panels) or pNC25085 (PCMV-EGFP-TMD-LifeAct; lower panels). Fluorescent images were taken 24 hours post transfection Scale bars, 10 μm (upper) and 10 μm (lower).
Photolytic cleavage of the retention domain enables mild light-inducible protein secretion
Having established that LifeAct effectively retains secretory vesicles, we next examined whether signal-induced disruption of the retention complex could trigger controlled protein secretion. To test this, we generated a POI-TMD-mMaple3-LifeAct construct, in which mMaple3, a photoconvertible fluorescent protein, serves as a light-responsive element. mMaple3 emits green fluorescence under basal conditions but undergoes irreversible photoconversion to a red-emitting form upon illumination with 405 nm light, accompanied by a cleavage at the 66-67 amino acid2,3 that were expected to release the tethered vesicles (Figure 2A).
To evaluate this design, HEK293T cells were transfected with pNC25088 (PCMV-IgK-Nluc-FS-TMD-mMaple3-LifeAct) and subjected to optical stimulation using a custom-built illumination system (see Hardware page for details; Figure 2B). Cells exposed to 405 nm light at a cycle of 10 s ON and 15 s OFF for 10 minutes exhibited markedly increased red fluorescence intensity relative to non-illuminated controls, confirming efficient photoconversion (Figure 2C). Reporter assays showed a modest but reproducible increase in NanoLuc secretion following 10 min light stimulation compared with dark controls (Figure 2D).
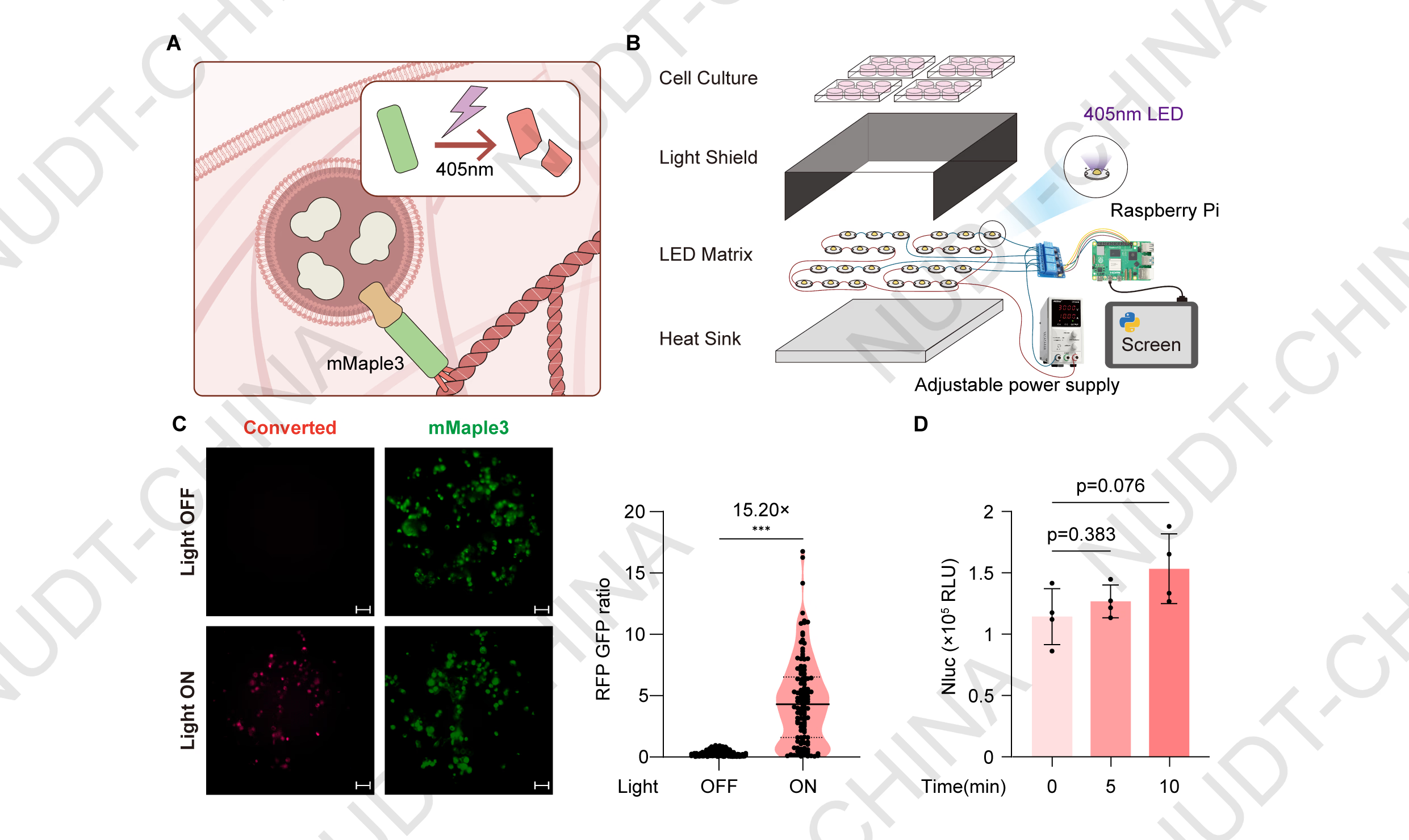
Figure 2.Photolysis of the mMaple3 retention module enables mild light-inducible protein secretion.
(A) Schematic illustration of the mMaple3-based secretion switch. Upon violet-light illumination (405 nm), mMaple3 undergoes photolysis, disrupting the LifeAct-mediated vesicle anchoring and triggering secretion.
(B) Exploded view of the custom optical stimulation device used for controlled light induction.
(C) Cellular Red Fluorescece/ Green Fluorescence intensity. Representative fluorescence images of pNC25088-transfected HEK293T cells maintained in the dark (control) or stimulated with violet light (Light). Fluorescent images were taken 48 hours post transfection. Scale bar, 50 µm. Quantification of single-cell red/green fluorescence ratios was performed using ImageJ. ***P < 0.001, two-tailed unpaired t-test.
(D) NanoLuc secretion levels under different stimulation durations. HEK293T cells were transfected with pNC25019 (PCMV-IgK-Nluc-mMaple3-LifeAct). 48 hours post-transfection, cells were illuminated with pulsed violet light (405 nm; 10 s ON/15 s OFF). Supernatants were collected before stimulation (0 min) and at 5 and 10 min after light exposure to quantify NanoLuc activity. Data represent mean ± SD from n = 4 independent experiments; p-value obtained by two-tailed unpaired t-test.
Notably, despite cells remaining morphologically intact, illumination caused a significant reduction in NanoLuc activity in cells transfected with the control construct pNC25089 (PCMV-IgK-Nluc) (Figure 3A-B), indicating that 405 nm light exposure was cytotoxic under these conditions.
Collectively, these findings suggest that while violet-light illumination introduces measurable cytotoxicity, photolysis of the mMaple3-LifeAct retention module enables mild, light-inducible secretion of the target protein, demonstrating proof-of-concept for signal-controlled post-translational secretion.
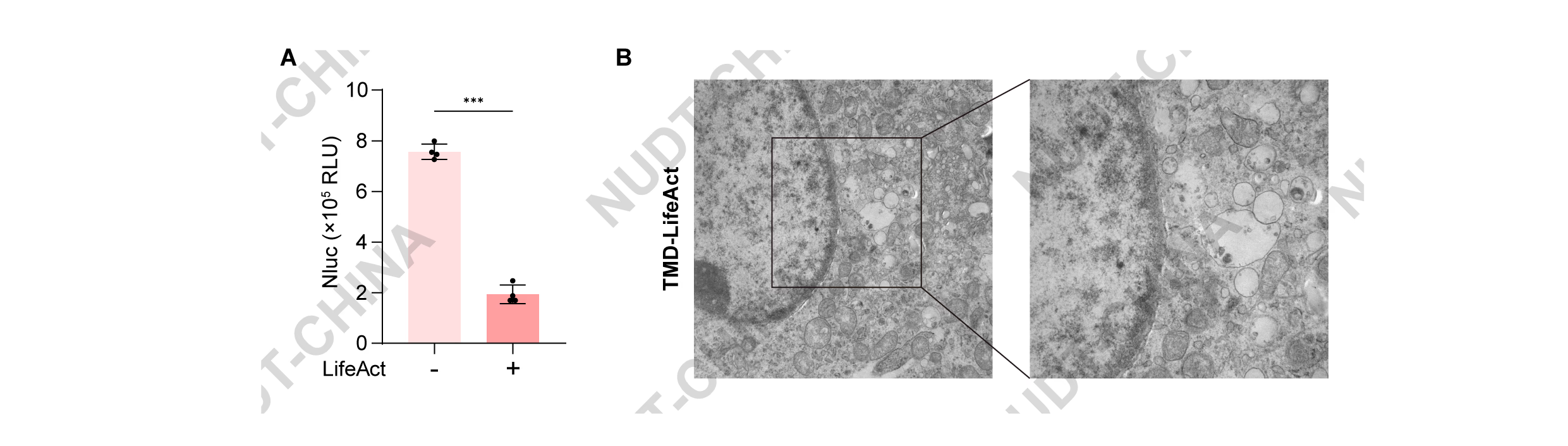
Figure 3.Violet-light illumination induces cytotoxicity and decreases constitutive secretion efficiency.
(A) Effect of violet-light stimulation on constitutive protein secretion. HEK293T cells transfected with pNC25089 (PCMV-IgK-Nluc) were illuminated with pulsed violet light (405 nm; 10 s ON/15 s OFF) 48 h post-transfection. NanoLuc activity in culture supernatants was measured before stimulation (0 min) and at 5 and 10 min after illumination. Data represent mean ± SD from n = 4 independent experiments.
(B) Representative phase-contrast images of HEK293T cells transfected with pNC25088 (PCMV-IgK-Nluc-FS-TMD-mMaple3-LifeAct) maintained in the dark or after violet-light stimulation (405 nm; 10 s ON/15 s OFF) for 15 min. Scale bar, 50 µm.
Light-controlled protein dissociation enables green- and near-infrared light-inducible secretion in optoSPARK
Given that violet-light illumination caused cytotoxicity, we next sought to engineer a secretion system responsive to longer-wavelength light, which penetrates tissue more effectively and minimizes phototoxic effects. Guided for insights from Dr. Human Practices investigations (see Human Practices page for details ), we reasoned that while most photolytic proteins respond to short-wavelength light, light-inducible protein dissociation could serve as an alternative regulatory mechanism. Based on this principle, we developed optoSPARK, a modular system that couples vesicle release to signal-induced protein dissociation under green or near-infrared (NIR) light (see Engineering page for design details).
For the NIR-responsive design, we replaced mMaple3 with the BphP1-PpsR2 interaction module, in which BphP1 homodimers dissociate upon illumination and subsequently bind to PpsR24. For green-light activation, we incorporated the CarH module, which aggregates in the dark and dissociates upon 540 nm light exposure5. In both systems, dissociation of the photoreactive proteins disrupts the LifeAct-mediated anchoring, leading to signal-induced vesicle release (Figure 4A, 4C).
Experimentally, HEK293T cells expressing either the NIR- or green-light system were illuminated under pulsed conditions for 2 hours (740 nm, 30 s ON / 210 s OFF for NIR; 540 nm, 30 s ON / 30 s OFF for green light). In both cases, NanoLuc secretion increased significantly compared with dark controls, indicating efficient light-triggered protein release (Figure 4B, 4D).
Together, these results demonstrate that protein dissociation-based control enables rapid and programmable secretion under low-energy light, effectively overcoming the cytotoxic limitations of violet-light-dependent systems. The optoSPARK framework thus establishes a versatile foundation for biocompatible, light-inducible post-translational regulation in mammalian cells.
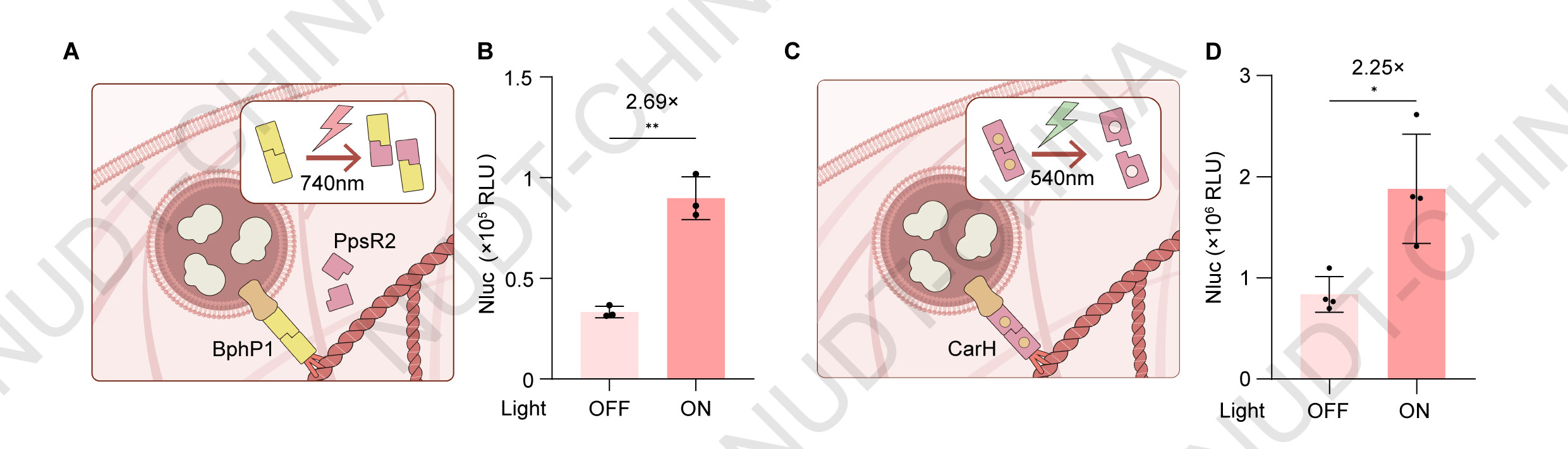
Figure 4.Signal-controlled protein dissociation enables green- and near-infrared light-inducible secretion in mammalian cells.
(A) Schematic illustration of the near-infrared (NIR)-responsive secretion switch. Upon 740 nm illumination, BphP1 homodimers dissociate and bind to PpsR2, releasing vesicles from LifeAct-mediated anchoring.
(B) NanoLuc secretion of HEK293T cells transfected with pNC25054 (PCMV-PpsR2), pNC25056 (PCMV-BphP1-LifeAct), and pNC25057 (PCMV-IgK-Nluc-FS-TMD-BphP1) in a 1:1:1 ratio. Cells were illuminated with pulsed NIR light (740 nm; 30 s ON / 210 s OFF) 48 hours post transfection, NanoLuc activity was measured immediately after illumination. Data represent mean ± SD from n = 4 independent experiments. ** 0.001 < P < 0.01, two-tailed unpaired t-test.
(C) Schematic illustration of the green-light-responsive secretion switch. CarH aggregates in the dark and dissociates upon 540 nm illumination, leading to vesicle release.
(D) NanoLuc secretion of HEK293T cells transfected with pNC25041 (PCMV-IgK-Nluc-FS-TMD-2×GSlinker-CarH) and pNC25043 (PCMV-LifeAct-CarH) in a 1:6 ratio. Cells were illuminated with pulsed green light (540 nm; 30 s ON / 30 s OFF) 48 hours post transfection, NanoLuc activity was measured immediately after illumination. Data represent mean ± SD from n = 4 independent experiments. * 0.01< P <0.05, two-tailed unpaired t-test.
Drug-responsive protein dissociation enables chemically inducible secretion in chemSPARK
Building on the success of light-inducible secretion via protein dissociation, we next sought to establish a system responsive to chemical inducers, enabling versatile and clinically translatable control of protein secretion. To achieve this, we designed chemSPARK, a modular secretion system regulated by drug-induced protein dissociation.
The design is based on the interaction between Binder (Apo-NS3a reader) and the hepatitis C virus protease NS3aH16,7. Previous studies have shown that this interaction can be rapidly disrupted by small-molecule inhibitors such as grazoprevir, which competes for the NS3a active site, leading to complex dissociation within minutes. Leveraging this dynamic behavior, we constructed a secretion switch driven by Binder-NS3a-mediated vesicle anchoring (Figure 5A).
To test this design, HEK293T cells were co-transfected with the pNC25091 (PCMV-Binder-LifeAct), and pNC25090 (PCMV-Nluc-TMD-NS3a). and treated with either DMSO (control) or grazoprevir 48 hours post-transfection. Compared with DMSO-treated controls, cells exposed to grazoprevir showed a mild but reproducible increase in NanoLuc secretion, confirming drug-inducible release (Figure 5B).
We next optimized the system by varying the copy numbers of Nluc and NS3a within the expression constructs. As expected, increasing the copy number of Nluc markedly enhanced grazoprevir-induced secretion, likely due to the accumulation of additional reporter protein within secretory vesicles (Figure 5C). Conversely, increasing the copy number of NS3a reduced basal secretion levels, thereby improving the overall fold induction. With the optimized copy configuration, we further evaluated whether the ratio between the actin-anchoring and secretion modules affected circuit performance. HEK293T cells were co-transfected with pNC25092 (PCMV-IgK-(Nluc)3-TMD-(NS3a)2) and either pNC25086 (PCMV-LifeAct-mCherry; control) or pNC25091 (PCMV-Binder-LifeAct). Optimal secretion was achieved at a 3:1 ratio, which provided a balance between expression strength and responsiveness (Figure 5D).
Together, these results demonstrate that chemSPARK enables chemically induced, post-translational control of protein secretion in mammalian cells. By employing a drug-responsive dissociation mechanism, this system provides a robust, modular, and clinically compatible platform for small-molecule-regulated therapeutic protein release.
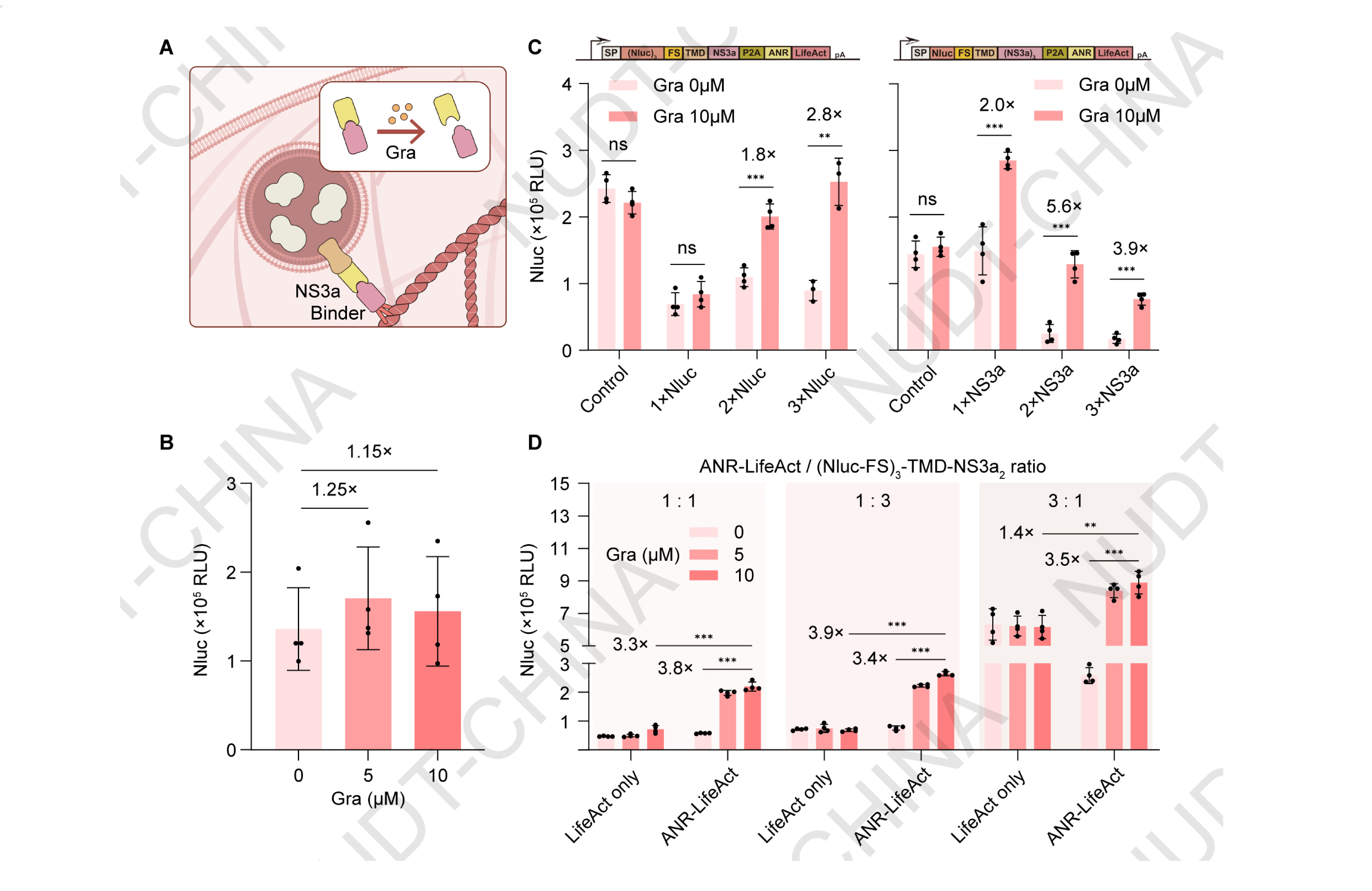
Figure 5.Chemical signal-induced protein dissociation enables drug-responsive secretion in chemSPARK.
(A) Schematic illustration of the secretion switch design based on grazoprevir-induced dissociation between NS3a and Binder. Under basal conditions, the NS3a-Binder interaction anchors secretory vesicles to the cytoskeleton, preventing release. Upon grazoprevir treatment, the complex dissociates, triggering vesicle release and protein secretion.
(B) NanoLuc secretion of HEK293T cells co-transfected with pNC25091 (PCMV-Binder-LifeAct) and pNC25090 (PCMV-Nluc-TMD-NS3a). Cells were treated with DMSO or grazoprevir at the indicated concentrations 48 hours post transfection, Nanoluc levels were quantified 2 hours post induction. Data represent mean ± SD from n = 4 independent experiments measured 2 h after stimulation.
(C) Effect of varying Nluc and NS3a copy numbers on secretion efficiency. HEK293T cells were transfected with either pNC25093 (PCMV-IgK-Nluc-TMD-NS3a-Binder-LifeAct), pNC25094 (PCMV-IgK-(Nluc)2-TMD-NS3a-Binder-LifeAct), pNC25095 (PCMV-IgK-(Nluc)3-TMD-NS3a-Binder-LifeAct), pNC25096 (PCMV-IgK-Nluc-TMD-(NS3a)2-Binder-LifeAct), or pNC25097 (PCMV-IgK-Nluc-TMD-(NS3a)3-Binder-LifeAct). Cells were treated with DMSO or grazoprevir 48 h post-transfection, and NanoLuc levels were quantified 2 h after stimulation. Data represent mean ± SD from n = 4 independent experiments. ** 0.001 < P < 0.01, two-tailed unpaired t-test.
(D) Optimization of transfection ratio between anchoring and secretion modules. HEK293T cells were co-transfected with pNC25092 (PCMV-IgK-(Nluc)3-TMD-(NS3a)2) and either pNC25086 (PCMV-LifeAct-mCherry; control) or pNC25091 (PCMV-Binder-LifeAct). Cells were treated with DMSO or grazoprevir 48 h post-transfection, and NanoLuc levels were quantified 2 h after stimulation. Data represent mean ± SD from n = 4 independent experiments. ** 0.001< P < 0.01, two-tailed unpaired t-test.
ChemSPARK exhibits rapid and dose-dependent secretion dynamics with faster activation than transcriptional control
Having established that chemSPARK enables chemically inducible secretion through drug-responsive protein dissociation, we next characterized its dose sensitivity and temporal dynamics to assess suitability for downstream applications.
To characterize chemSPARK, HEK293T cells were co-transfected with pNC25091 (PCMV-LifeAct-mCherry-Binder) and pNC25092 (PCMV-(Nluc-FS)3-TMD-(NS3a)2) at a 3:1 ratio, and treated 48 h post-transfection with varying concentrations of grazoprevir (0-10 µM) or an equivalent volume of DMSO as control. NanoLuc levels were measured 2 h after drug addition. Results showed a clear dose-dependent induction of NanoLuc secretion, reaching a 40.1-fold increase at 10 µM grazoprevir (Figure 6A). Time-course analysis further revealed a rapid and sustained activation of the optimized system, with NanoLuc levels elevated 2.7-fold, 12.7-fold, and 12.1-fold at 1, 2, and 4 h post-stimulation, respectively (P = 0.000082 at 1 h; P <0.000001 at 2 and 4 h). These findings demonstrate that chemSPARK responds rapidly and proportionally to drug stimulation, achieving both high sensitivity and tunable control within hours of induction.
In contrast, a conventional transcriptional activation system---comprising pNC25046 (PCMV-Binder-TetR-NLS-VP64), pNC25048 (PCMV-IgK-Fluc-TMD-NS3a), and pNC25098 (PTCE-IgK-Nluc), exhibited delayed protein secretion, with NanoLuc induction detectable only 12 h post-stimulation. This clear difference underscores the superior speed and responsiveness of chemSPARK as a post-translational genetic switch, capable of near-instantaneous small-molecule-controlled protein release (Figure 6B).
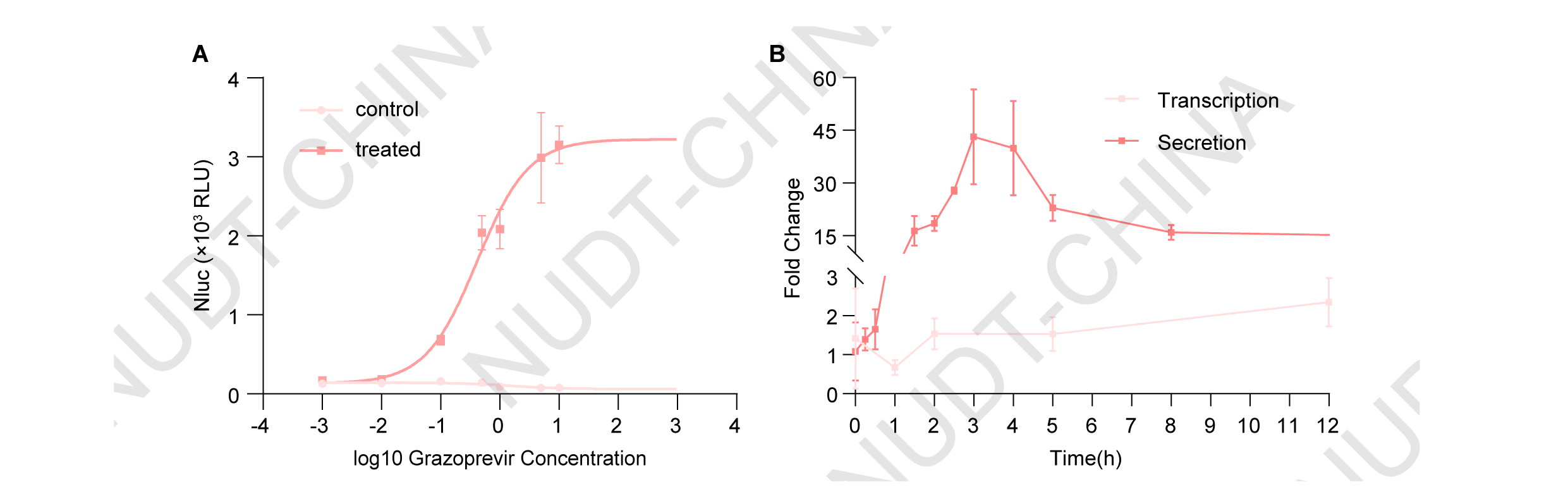
Figure 6.chemSPARK exhibits rapid and dose-dependent secretion dynamics compared with a transcriptional activation system.
(A) Dose-dependent secretion response to Grazoprevir treatment(0-10 µM). Data presents mean ±SD, n = 3 independent experiments, fitted to a symmetric sigmoid curve.
(B) Kinetics of grazoprevir-inducible NanoLuc expression comparing chemSPARK with a transcriptional activation system. For the transcriptional system, HEK293T cells were co-transfected with pNC25046 (PCMV-Binder-TetR-NLS-VP64), pNC25048 (PCMV-IgK-Fluc-TMD-NS3a), and pNC25098 (PTCE-IgK-Nluc). For the secretion system, cells were co-transfected with pNC25092 (PCMV-(Nluc-FS)3-TMD-(NS3a)2) and pNC25091 (PCMV-LifeAct-mCherry-Binder). In both systems, 10 µM grazoprevir was added at the indicated time points (6 h post-transfection for transcriptional and 48 h for secretion), and NanoLuc levels were monitored over the following 48 h and 12 h, respectively. Data represent mean ± SD of fold changes calculated as the ratio of NanoLuc levels in grazoprevir-treated versus untreated samples (n = 3 independent experiments).
 Description
Description
 Design
Design
 Notebook
Notebook
 Results
Results
 Basic Parts
Basic Parts
 Composite Parts
Composite Parts
 Parts Collection
Parts Collection
 Education
Education
 Art Gallery
Art Gallery
 Implementation
Implementation
 Attributions
Attributions
 Collaboration
Collaboration