

To achieve the production of astaxanthin in Escherichia coli through synthetic biology, we established a cell factory by engineering a plasmid that contained astaxanthin biosynthesis pathway and introducing it into E. coli. Seven genes including HpCrtE, PanCrtB, PanCrtI, idi, PagCrtY, HpCrtZ, and BreCrtW were chosen and synthesized by Nanjing GenScript Biotech Co., Ltd., and then inserted into pET-duet-1 vector to construct plasmid pET-ast. According to our design, this cell factory is controlled by dual T7 promoters. Using the common commercial expression plasmid pET-duet-1 as the backbone, HpCrtE, PanCrtB, and PanCrtI were inserted into the multiple cloning sites of the first T7 promoter, while idi, PagCrtY, HpCrtZ, and BreCrtW were inserted into the multiple cloning sites of the second T7 promoter. This resulted in the pET-ast plasmid, which was delivered in the form of glycerol stock cultures. We also tried to improve the catalytic activity of HpCrtZ through protein structure analysis, alanine scanning, and site directed mutagenesis. Based on the functional analysis of HpCrtZ mutants, we obtained four HpCrtZ mutants that could significantly increase astaxanthin production. Using synthetic biology techniques to design cell factories for the production of astaxanthin can reduce production costs and ensure astaxanthin activity, which is of great significance for industries such as aquaculture and cosmetics. Our business plan has beneficial results in expanding the application of astaxanthin and ensuring public health.
We designed and optimized the astaxanthin biosynthetic pathway and registered five new BioBricks and one Composite part. We provide a better biosynthetic pathway for astaxanthin, in which we intruduced HpCrtE (BBa_25QSWB7M) and HpCrtZ (BBa_25ESIC8B) from Haematococcus pluvialis that is the highest astaxanthin content in nature.
| Part ID | Name | Type | Part Description |
|---|---|---|---|
| BBa_25QSWB7M | HpCrtE from Haematococcus pluvialis | Protein coding sequence (new basic part) | The GGPP synthase (CrtE) catalyzes the formation of geranyl geranyl pyrophosphate (GGPP) from farnesyl pyrophosphate (FPP) and it involves in the synthesis of astaxanthin. |
| BBa_25HIN5VJ | PanCrtB from Pantoea ananatis | Protein coding sequence (new basic part) | It catalyzes the production of phytoene from geranylgeranylpyrophosphate (GGPP) in the presence of lycopene synthase (CrtB) and it involves in the synthesis of astaxanthin. |
| BBa_25ITTDLS | PanCrtI from Pantoea ananatis | Protein coding sequence (new basic part) | It catalyzes the formation of lycopene from phytoene in the presence of phytoene desaturase (CrtI) and it involves in the synthesis of astaxanthin. |
| BBa_25ESIC8B | HpCrtZ from Haematococcus pluvialis | Protein coding sequence (new basic part) | β - carotene hydroxylase (CrtZ) catalyzes the formation of astaxanthin from canthaxanthin and it involves in the synthesis of astaxanthin. |
| BBa_250V9MEZ | BreCrtW Brevundimonas sp. SD212 | Protein coding sequence (new basic part) | β - carotene ketolase (CrtW) catalyzes the formation of zeaxanthin from β - carotene and it involves in the synthesis of astaxanthin. |
| BBa_251FM2MG | pET-ast | Composite part (new plasmid) | pET-ast was constructed in our lab based on pET-Dute-1 vector (BBa_K5492101), which contained HpCrtE from Haematococcus pluvialis (BBa_25QSWB7M), PanCrtB from Pantoea ananatis (BBa_25HIN5VJ), PanCrtI from Pantoea ananatis (BBa_25ITTDLS), idi from Escherichia coli (BBa_K3166058), PagCrtY from Pantoea agglomerans (BBa_K3279003), HpCrtZ from Haematococcus pluvialis (BBa_25ESIC8B), BreCrtW from Brevundimonas sp. SD212 (BBa_250V9MEZ). After transforming into E.coli, pET-ast own the ability to synthesize astaxanthin. |
We consrtucted and optimised the astaxanthin synthesis pathway by intruducing HpCrtE(BBa_25QSWB7M), HpCrtZ(BBa_25ESIC8B) from Haematococcus pluvialis. Five new basic parts including BBa_25QSWB7M, BBa_25HIN5VJ, BBa_25ITTDLS, BBa_25ESIC8B, BBa_250V9MEZ, BBa_251FM2MG were registered that would be helpful to the other researchers. We also provided the additional information to the old part of backbone (BBa_K5492101), which would help the other people express mutiple genes with pET-Duet-1 vector. We believe that people can express up to 10 genes using pET-Duet-1 due to two T7 promoter that can express in high levle when inducing with IPTG. The CrtY gene is very important to the astaxanthin synthesis because it can convert lycopene to β-carotene. Howover, little information was provide in part registration. We added a little information to the old part (BBa_K3279003) including protein structure, classification and phylogenetic tree. We believed that it would help for the others. The CrtE catalyzes the formation of geranyl geranyl pyrophosphate (GGPP) from farnesyl pyrophosphate (FPP) and it involves in the synthesis of astaxanthin. It is very important to increase astaxanthin production. However, we found little information in iGEM part registration. Hence, we provided some information to the old part -CrtE (BBa_K2149000) including its function, classification, gene structure and so on. Finally, we constructed a composit part (BBa_251FM2MG) named pET-ast that contained HpCrtE, PanCrtB, PanCrtI, idi, PagCrtY, HpCrtZ, and BreCrtW. We tested the functions of pET-ast in E. coli and results showed that it can produce astaxanthin meaning that we succeeded in constructing a new astaxanthin synthesis pathway. This work would provide a new astaxanthin synthesis pathway to the researchers when they want to improve the astaxantin production.
Through protein structure prediction, substrate molecular docking, and site-directed mutagenesis, we have obtained four HpCrtZ mutants with enhanced catalytic activity. This provides an active site for the targeted engineering of HpCrtZ and supplies a key enzyme with higher catalytic activity for astaxanthin synthesis.
| Part ID | Name | Type | Part Description |
|---|---|---|---|
| BBa_255CQOV8 | HpCrtZ ILE102ALA mutant | Protein coding sequence (new basic part) | The ILE at position 102 is transformed into ALA in HpCrtZ mutant , improving the efficiency of converting canthaxanthin into astaxanthin. |
| BBa_25YM5299 | HpCrtZ SER96ALA mutant | Protein coding sequence (new basic part) | The SER at position 96 is transformed into ALA in HpCrtZ mutant , improving the efficiency of converting canthaxanthin into astaxanthin. |
| BBa_259A010E | HpCrtZ CYS191ALA mutant | Protein coding sequence (new basic part) | The CYS at position 191 is transformed into ALA in HpCrtZ mutant , improving the efficiency of converting canthaxanthin into astaxanthin. |
| BBa_255X8X9N | HpCrtZ THR213ALA mutant | Protein coding sequence (new basic part) | The THR at position 213 is transformed into ALA in HpCrtZ mutant , improving the efficiency of converting canthaxanthin into astaxanthin. |
Through protein structure analysis and molecular docking with substrates, the substrate pocket of HpCrtZ was identified, and key amino acid sites were determined. The binding sites of β-carotene hydroxylase with canthaxanthin were predicted to position at TYR88, TRP152, HIS165, GLU174, ASN176, ASP177, PHE179, ALA180, ASN183, GLY216, TYR219, MET220, HIS223, ASP224, ARG230. This finding provides a valuable model for subsequent researchers to conduct protein design on HpCrtZ. Targeting the active site for modification offers higher efficiency and a greater chance of obtaining valuable mutants, which can inspire subsequent efforts in engineering astaxanthin synthases.
The amino acids of the biding pocket were mutated to alanine and their binding energies with substrates were detected. Compared with the unmutated β-carotene hydroxylase, the binding energies of the β-carotene hydroxylase mutants ILE102ALA (BBa_255CQOV8), SER96ALA (BBa_25YM5299), CYS191ALA (BBa_259A010E), and THR213ALA(BBa_255X8X9N) to canthaxanthin were significantly lower than the average value, showing that they have a role in enhancing β-carotene hydroxylase activity with potential. Of these mutants, HpCrtZ (CYS191ALA, BBa_259A010E) showed the highest catalytic activity improving astaxanthin production by 1.28-fold. Our findings would help the people to increase astaxanthin production in the future.
Learn more in Parts.
By utilizing metabolic engineering techniques, researchers have successfully produced astaxanthin in bacteria and yeast. This fermentation-based production method is not only controllable and easily scalable but also avoids the high costs and safety concerns typically associated with extraction from algae or chemical synthesis.
Chemically synthesized astaxanthin contains numerous impurities and cannot be used in products for human consumption. Astaxanthin derived from Haematococcus pluvialis meets safety requirements for human use and can be applied in cosmetics and health supplements, but its production costs are high. While astaxanthin produced using E. coli offers high yield, it is currently not approved for direct use in human-related products. On February 7, 2023, Taiwan's Ministry of Health and Welfare issued amendments to regulations on the use and labeling of the food ingredient 2'-fucosyllactose produced by genetically modified E. coli fermentation. With advancing technology, astaxanthin produced using E. coli may also become applicable in related products in the future. Additionally, the group standard "Technical Requirements for the Synthesis of Natural Astaxanthin by Microbial (Phaffia rhodozyma) Fermentation," issued by the China International Agrotechnical and Economic Association, has been officially implemented, suggesting that yeast may also be explored as a potential host for astaxanthin production.
The cell factory can produce highly active astaxanthin with a configuration identical to the natural form. Its antioxidant activity is significantly superior to chemically synthesized products, and some studies even indicate it outperforms natural extracts. This means that future health supplements and functional foods will be more effective. Compared with the traditional extraction method from Haematococcus pluvialis—which involves long cultivation cycles and high extraction costs—microbial fermentation can improve efficiency and scale, potentially significantly reducing production costs. This would make high-quality astaxanthin products more affordable for a broader range of consumers. Astaxanthin is developing rapidly in the global market and is becoming increasingly popular among consumers (Figure 1). With lower costs, astaxanthin can be more widely used in feed, cosmetics, as well as in common foods and beverages, becoming integrated into various aspects of daily life.
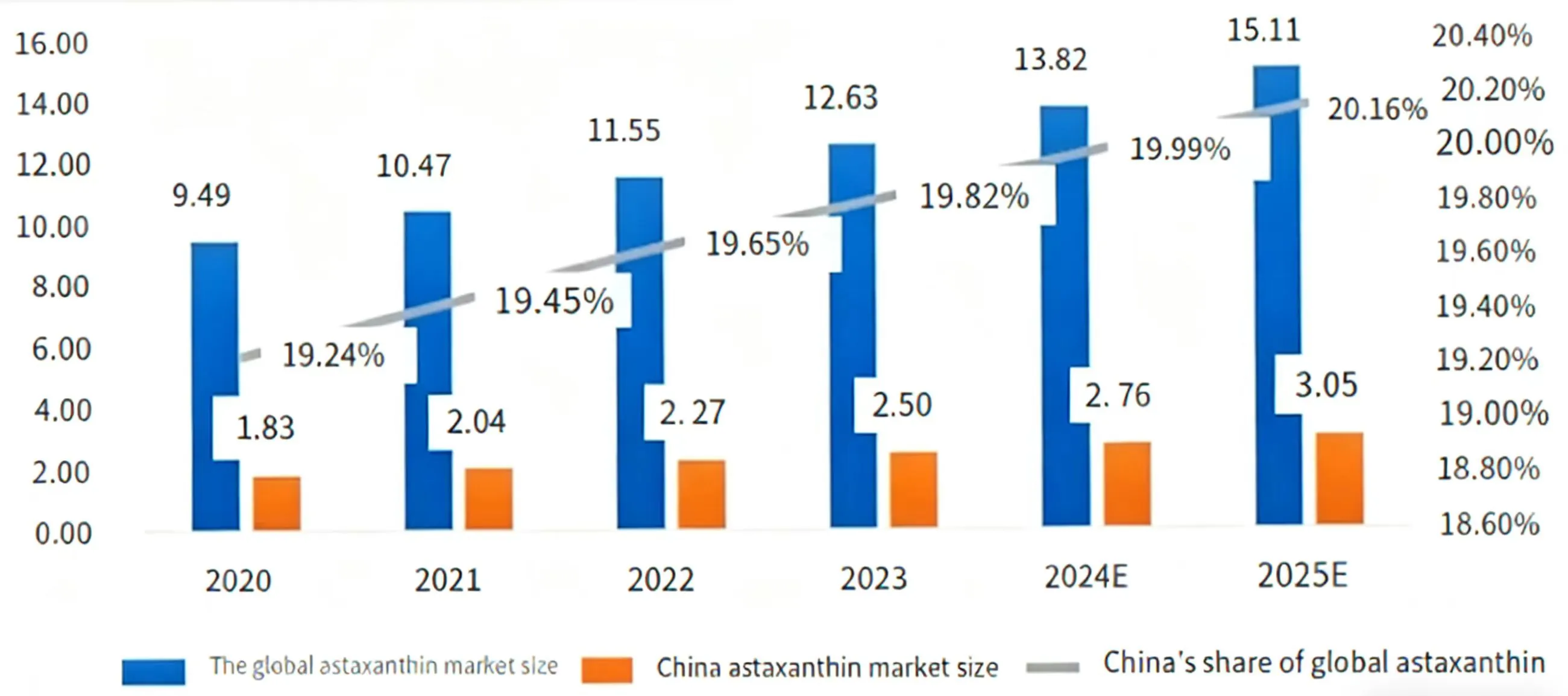 Figure 1. Global and Chinese astaxanthin market size from 2020 to 2025 (Unit: 100 million yuan)
Figure 1. Global and Chinese astaxanthin market size from 2020 to 2025 (Unit: 100 million yuan)
In our business plan, we can produce astaxanthin as the industrial raw material which will be beneficial for cosmetics and other industries. We can also use astaxanthin to make facial mask as a market product. According to insights from traditional Chinese opera performers, astaxanthin can be utilized to create face cream essence that prevent skin oxidative aging, as well as to produce skin friendly pigments for facial coloring, indicating its significant future potential.