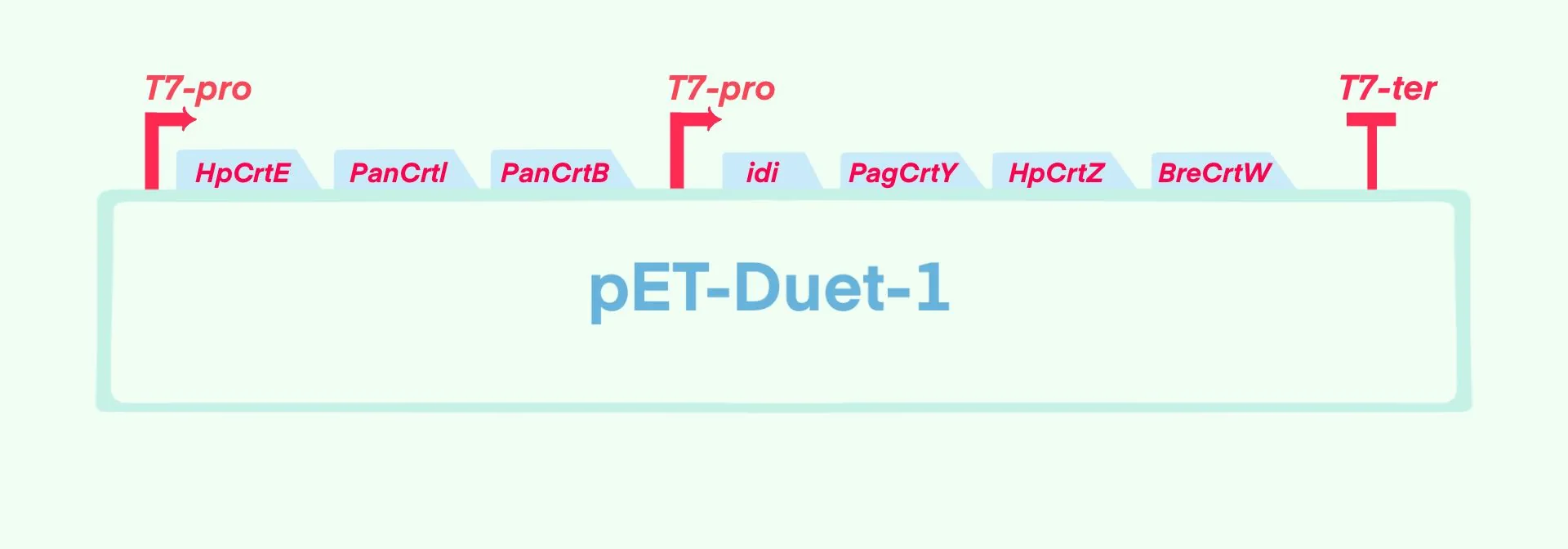

To achieve astaxanthin production in Escherichia coli ( E. coli), we constructed a plasmid by integrating seven genes essential for astaxanthin synthesis and introduced this plasmid into E. coli, thereby establishing a cell factory for astaxanthin biosynthesis.
As designed, this cell factory is controlled by dual T7 promoters and uses the common commercial expression plasmid pET-Duet-1 as the backbone. Specifically, the genes HpCrtE, PanCrtB, and PanCrtI were inserted into the multiple cloning site (MCS) of the first T7 promoter, while idi, PagCrtY, HpCrtZ, and BreCrtW were inserted into the MCS of the second T7 promoter. Through this process, the plasmid pET-ast was obtained. Subsequently, steps including resistance screening, scale-up culture, IPTG induction, and product detection were implemented to enhance the feasibility of the experiment.
In addition, we performed alanine scanning to mutate the amino acids in the substrate-binding pocket of β-carotene hydroxylase. From this, four mutants with significantly increased astaxanthin yield were screened out, with the highest yield improvement reaching 1.28-fold.
Seven astaxanthin synthesis-related genes were obtained through literature review [1], namely HpCrtE (BBa_25QSWB7M), PanCrtB (BBa_25HIN5VJ), PanCrtI (BBa_25ITTDLS), idi (BBa_K3166058), PagCrtY (BBa_K3279003), HpCrtZ (BBa_25ESIC8B), and BreCrtW (BBa_250V9MEZ).
The seven essential genes for astaxanthin synthesis were organized into two expression modules, which were inserted into the restriction enzyme sites of the pET-Duet-1 vector (BBa_K5492101) respectively. The astaxanthin synthesis pathway was thus integrated, and finally the pET-ast (BBa_251FM2MG) was obtained.
In E.coli BL21 (DE3), the T7 promoter was employed. Under induction by IPTG, the expression efficiency of these genes was significantly improved, which further facilitated the high-rate and high-yield production of astaxanthin.
| Part ID | Name | Type | Part Description |
|---|---|---|---|
| BBa_25QSWB7M | HpCrtE from Haematococcus pluvialis [2] | Protein coding sequence (new basic part) | The GGPP synthase (CrtE) catalyzes the formation of geranyl geranyl pyrophosphate (GGPP) from farnesyl pyrophosphate (FPP) and it involves in the synthesis of astaxanthin. |
| BBa_25HIN5VJ | PanCrtB from Pantoea ananatis | Protein coding sequence (new basic part) | It catalyzes the production of phytoene from geranylgeranylpyrophosphate (GGPP) in the presence of lycopene synthase (CrtB) and it involves in the synthesis of astaxanthin. |
| BBa_25ITTDLS | PanCrtI from Pantoea ananatis | Protein coding sequence (new basic part) | It catalyzes the formation of lycopene from phytoene in the presence of phytoene desaturase (CrtI) and it involves in the synthesis of astaxanthin. |
| BBa_K3166058 | idi | Protein coding sequence | It is the basic part of (rbs-idi-rbs-ispA-rbs-YSS)(BBa_K3166058) and it plays an important role in improving the yield of squalene with YSS. |
| BBa_K3279003 | lycopene beta-cyclase (CrtY) | Protein coding sequence | This part is a lycopene beta-cyclase which transforms lycopene into β-carotene. The enzyme is a non-redox flavoprotein, containing FADH2 that is used for stabilization of a transition state. Lycopene has a psi-end group at both ends. When acting on one end, the enzyme forms gamma-carotene. When acting on both ends it forms β-carotene. It also acts on neurosporene to give beta-zeacarotene. This sequence is optimized for use in E.coli. |
| BBa_25ESIC8B | HpCrtZ from Haematococcus pluvialis [3] | Protein coding sequence (new basic part) | β - carotene hydroxylase (CrtZ) catalyzes the formation of astaxanthin from canthaxanthin and it involves in the synthesis of astaxanthin. |
| BBa_250V9MEZ | BreCrtW Brevundimonas sp. SD212 | Protein coding sequence (new basic part) | β - carotene ketolase (CrtW) catalyzes the formation of zeaxanthin from β - carotene and it involves in the synthesis of astaxanthin. |
| BBa_K5492101 | pET DueT-1 | Plasmid backbone | pET DueT-1 plasmid used for expression of Histamin-N-Methyltransferase and Diamine Oxidase in multiple E.coli chassis. |
| BBa_251FM2MG | pET-ast | Composite part (new plasmid) | pET-ast was constructed in our lab based on pET Duet-1 vector (BBa_K5492101), which contained HpCrtE from Haematococcus pluvialis (BBa_25QSWB7M), PanCrtB from Pantoea ananatis (BBa_25HIN5VJ), PanCrtI from Pantoea ananatis (BBa_25ITTDLS), idi from Escherichia coli (BBa_K3166058), PagCrtY from Pantoea agglomerans (BBa_K3279003), HpCrtZ from Haematococcus pluvialis (BBa_25ESIC8B), BreCrtW from Brevundimonas sp. SD212 (BBa_250V9MEZ). After transforming into E.coli, pET-ast own the ability to synthesize astaxanthin. |
Targeting the pocket structure of β-carotene hydroxylase, we performed alanine scanning, where amino acids in the pocket region were mutated to alanine. By analyzing changes in the binding energy between β-carotene hydroxylase mutants and canthaxanthin, we screened out four β-carotene hydroxylase mutants.Hydroxylase mutants with reduced binding energy, namely HpCrtZILE102ALA (BBa_255CQOV8), HpCrtZSER96ALA (BBa_25YM5299), HpCrtZCYS191ALA (BBa_259A010E), and HpCrtZTHR213ALA (BBa_255X8X9N). The results showed that reducing the binding energy promoted the binding of canthaxanthin to the β-carotene hydroxylase mutants, leading to a significant increase in astaxanthin yield, with the highest yield being 1.28 times that before the mutation.
| Part ID | Name | Type | Part Description |
|---|---|---|---|
| BBa_255CQOV8 | HpCrtZILE102ALA mutant | Protein coding sequence (new basic part) | The ILE at position 102 is transformed into ALA in HpCrtZ mutant , improving the efficiency of converting canthaxanthin into astaxanthin. |
| BBa_25YM5299 | HpCrtZSER96ALA mutant | Protein coding sequence (new basic part) | The SER at position 96 is transformed into ALA in HpCrtZ mutant , improving the efficiency of converting canthaxanthin into astaxanthin. |
| BBa_259A010E | HpCrtZCYS191ALA mutant | Protein coding sequence (new basic part) | The CYS at position 191 is transformed into ALA in HpCrtZ mutant , improving the efficiency of converting canthaxanthin into astaxanthin. |
| BBa_255X8X9N | HpCrtZTHR213ALA mutant | Protein coding sequence (new basic part) | The THR at position 213 is transformed into ALA in HpCrtZ mutant , improving the efficiency of converting canthaxanthin into astaxanthin. |
[1] Wang, W., Li, Y., Hu, Z., et al. Genome mining of astaxanthin biosynthetic genes from Sphingomonas sp. ATCC 55669 for heterologous overproduction in Escherichia coli. biotechnol. j. 11, 228-237 (2016).
[2] Huang D, Liu W, Li A, Wang C, Hu Z. Discovery of geranylgeranyl pyrophosphate synthase (GGPPS) paralogs from Haematococcus pluvialis based on Iso-Seq analysis and their function on astaxanthin biosynthesis. Mar Drugs. 2019;17:696.
[3] Huang D , Liu W , Li A ,et al.Cloning and identification of a novel β-carotene hydroxylase gene from Haematococcus pluvialis and its function in Escherichia coli[J].Algal Research, 2021, 55(22):102245.